Abstract
Prions are infectious proteins. In fungi, prions correspond to non-Mendelian genetic elements whose mode of inheritance has long eluded explanation. The [Het-s] cytoplasmic genetic element of the filamentous fungus Podospora anserina, was originally identified in 1952 and recognized as a prion nearly half a century later. The present Chapter will attempt to describe the work on [Het-s] from a historical perspective. The initial characterization and early genetic and physiological studies of [Het-s] are described together with the isolation of [Het-s] encoding gene. More recent work that led to the construction of a structural model for this prion is also discussed.
The Discovery of “Petit s”
[Het-s] is a prion of the filamentous fungus Podospora anserina. [Het-s] was first identified as a non-Mendelian genetic element in 1952 by George Rizet, the founder of the french fungal genetics school. He discovered [Het-s] during the genetic dissection of a phenomenon typical of filamentous fungi and termed heterokaryon incompatibility.Citation1 Filamentous fungi grow as an interconnected network of filaments forming a syncytial structure (). Fusion of somatic cells readily occurs between strains, leading to the spontaneous formation of vegetative heterokaryons.Citation2 Many if not all organisms forming somatic chimeras posses genetic systems that allow them to distinguish somatic self from nonself. Fungi are no exception in that regard.Citation3 It is believed that the role of these systems is to prevent different forms of somatic cell parasitism. Viability and fitness of fungal heterokaryons is thus genetically controlled by a set of genes termed het genes.Citation4 het genes exist as two or more polymorphic allelic variants and only heterokaryons in which both nuclear components have compatible het gene constitution are viable. A genetic difference at any of 9 het loci found in Podospora leads to an incompatibility reaction resulting in severe growth inhibition and cell death of the heterokaryotic cells. Heterokaryon incompatibility systems also act as barriers limiting the transmission of deleterious cytoplasmic replicons such as mycoviruses or senescence plasmids. Transmission of such replicons between incompatible strains is reduced or abolished. In Podospora, the incompatibility reaction can easily be detected at the macroscopic level by confronting strains of solid medium. An incompatible interaction results in the formation of an abnormal contact line termed barrage. Thus wild-type strains can easily be classified into so-called vegetative compatibility groups (VCG), strains that are compatible are grouped within the same VCG.
The story of [Het-s] begins when Georges Rizet observed formation of a barrage between strains that where originally compatible (i.e., belonging to the same compatibility group).Citation1 Rizet designated these strains s and S (). To try to understand the event that led to emergence of this new incompatibility, he crossed s and S and noted a very unorthodox segregation of the parental phenotypes. Indeed, he recovered an expected 50% S progeny but no s progeny (). Instead the remaining strains displayed a novel phenotype that he designated sS; sS strains are compatible both with s and S. This nomenclature was meant to stress that the s character had been modified by its interaction with S. He then observed a phenomenon that he called “réversion” by which sS strains spontaneously reacquire the s phenotype. This reversion, he found, is an invasive phenomenon that rapidly spreads to the whole thallus and is transmitted to other sS strains by simple contact. He also described that the s and sS characters display maternal inheritance so that in a s x sS cross the progeny will uniformly display the phenotype of the maternal parent, the one that is contributing the cytoplasm to the zygote (). Rizet concluded from these studies that s is a cytoplasmic heritable particle that can be inactivated by the S factor. His exact words are that s strains contain a particle that is absent or exists in a modified form in sS strains.
Janine Beisson, as a student of Rizet, then examined the characteristic of the sS to s reversion. She showed that the reversion process requires cytoplasmic contact as occurring during hyphal fusion.Citation5 She measured the rate of the reversion process (the rate of propagation of the s element in a sS mycelium) and showed that this process is about ten times faster than the radial growth rate of the fungus (that is up to 70 mm per day). Janine Beisson was also able to show that the s element can be lost vegetatively without the intervention of the S factor when mycelium is regenerated from cellular structures containing very little cytoplasm. Léon Belcour later showed that the s element can also be lost during generation of isolated cells by protoplast formation, meaning that upon regeneration of protoplasts a small fraction of the regenerating mycelia become sS.Citation6 He made the reasonable inference that “The simplest interpretation of these results is that passive and random distribution of cytoplasm occurs during protoplast formation. Those protoplasts receiving the s cytoplasmic factor would yield s mycelia. Those not receiving it would yield sS mycelia”. On the basis of this line of reasoning, he estimated that there are 1.8 to 4.6 s units per protoplast or 7 to 17 s units per 1000 µCitation3 which is about similar to the number of nuclei.
By then the genetics of the s element were exquisitely described but naturally the physical basis of this non-Mendelian genetic element remained totally elusive. Still the “particular” nature of s had already been well recognized in those clever and careful genetic and physiological approaches. Janine Beisson proposed in 1962 that the s element regulates its own synthesis. Such a positive feed-back loop could explain the cytoplasmic transmission and maternal inheritance of the s trait.
Molecular Cloning of het-s and het-S, Some Answers, More Puzzles
In the late 1980s the development of a DNA transformation procedure for Podospora permitted the molecular characterisation of s and S genes. Joël Bégueret (another former student of Rizet) undertook the molecular cloning of s making use of an inactive allele of the s locus termed sX identified in a natural isolate of P. anserina. Strains carrying the sX allele are compatible both with s and S strains and cannot be converted to the s phenotype after contact with a wild-type s strain.Citation7,Citation8 Soon after its rebirth in the molecular age, s was rebaptised to conform to the nomenclature of the other fungal heterokaryon incompatibility genes. The s gene became het-s while the s and sS phenotypes became [Het-s] and [Het-s*] respectively ().
The het-s gene was cloned using a functional complementation approach. A het-sX strain was transformed with a het-s genomic DNA library and transformants were scored for appearance of the [Het-s] phenotype.Citation7 This approach allowed the cloning of the het-s and het-S alleles. Both alleles were found to encode proteins of 289 amino acids in length differing by 13 amino acid residues. het-s and het-S knock-out strains are both viable and fully fertile.Citation8 The het-sX allele derives from the het-s allele and contains a 46 bp duplication in the open reading frame leading to a premature stop codon at position 165.Citation9 The existence of a natural isolate in which het-s is in a pseudogene state might suggest that the het-s activity is not critical for survival even in the wild.
In order to identify which of the 13 polymorphic positions are responsible for the het-s and het-S allele specificities, Carole Deleu carried out an extensive mutational analysis.Citation9 She thus found that a single amino acid substitution is sufficient to turn a HET-S protein into a protein of the HET-s specificity. Amino acid 33 is a histidine in HET-S and a proline in HET-s. A H33P point mutation turns HET-S into a protein of HET-s specificity. In other words a strain expressing the HET-S 33P mutant is no longer incompatible with [Het-s] but instead becomes incompatible with [Het-S]. This means that in this particular case incompatibility is triggered by interaction of two proteins that differ by a single amino acid (!). In addition, as wild-type HET-s, this mutant strain can display the alternate [Het-s*] and [Het-s] states. Many other mutations including deletion of H33 turn HET-S to the HET-s specificity. The reciprocal specificity switch requires a double mutation in positions 23 and 33. In other words, HET-s D23A P33H behaves as HET-S. During the course of this work, Carole Deleu also characterized a remarkable allele of het-s, het-s 33H. Strains carrying this allele express the [Het-S] phenotype but can acquire the [Het-s] state after contact with a wild-type [Het-s] strain. Existence of this schizophrenic allele shows that the [Het-s] and [Het-S] states can be expressed alternatively from the same allele. So in addition to the [Het-s*] to [Het-s] transition there is the possibility of a second epigenetic switch from [Het-S] to [Het-s]. One wonders how these mutant strains manage the crisis situation that occurs during this switch as this transition necessarily leads to a transitory self-incompatibility.
The next puzzle brought forth by the molecular cloning of het-s and het-S was that of the evolutionary history of these alleles. Because Rizet saw the sudden emergence of the s and S characters, he was convinced that one allele derived from the other by a spontaneous mutation that occurred in the lab. The molecular cloning of the alleles clearly contradicted this view; the het-s and het-S alleles are too divergent for that. In addition, divergence between het-s and het-S is not limited to base substitutions in the ORFs. The het-s allele (but not het-S) contains the scar of a transposable element called REPA in its promoter region and in addition the 3′ region of the het-s locus contains a region of DNA of several kilobases that is totally absent from the genome of the S strain.Citation7–Citation10 Several french wild-type isolates of P. anserina have been analysed at the het-s locus and both allelic types were found to be well represented. The striking observation was that all het-s alleles are identical to the base pair.Citation9 The same is true for all het-S alleles. It appears that two discrete highly divergent alleles are fixed in the population with no frequent intermediate type. We have yet to come up with a convincing evolutionary scenario that could account for these observations. What is clear is that polymorphism at het-s is ancient.
Identification of [Het-s] as a Prion Form of the Het-s Protein
Once antibodies to HET-s were developed it was determined that the HET-s protein is present both in [Het-s*] and [Het-s] strains. In fact the protein is more abundant in [Het-s*] than in [Het-s] strains;Citation11 electrophoretic mobility of HET-s is identical in [Het-s] and [Het-s*]. At that point, a positive feed-back loop model on the protein synthesis per se had to be rejected. Alternatively one could envision that HET-s was capable of self-activation at the post-translational level and that this post-translational modification would not alter electrophoretic mobility in SDS-PAGE.
The prion hypothesis for [Het-s] is considered by Carole Deleu in her thesis manuscript defended in 1993 and it was also suggested in a letter send to Joël Bégueret by a perspicacious reader of a 1993 paper on het-s mutants. But the development of the prion hypothesis for [Het-s] had to await the remarkable demonstration of the existence of yeast prions by Reed Wickner.Citation12 It then became evident that the properties of the [Het-s] system could be explained in a model stating that the [Het-s] non-Mendelian genetic element corresponds to the prion form of the HET-s protein. It was shown that over-expression of the het-s gene increases spontaneous appearance of [Het-s] and that HET-s can adopt a protease resistant state in [Het-s] strains. Interestingly, Joël Bégueret showed that conversion of a mycelium from the [Het-s*] to the [Het-s] state can occur in the presence of cycloheximide and thus does apparently not require de novo protein synthesis.Citation11 This observation echoes the recent elegant demonstration that conversion of the Sup35 protein to the prion state can occur from mature protein.Citation13
The [Het-s] prion however displays an important genetic difference when compared to the prototypal yeast [URE3] and [PSI+] prions. [URE3] and [PSI+] are detected as total or partial loss of function of Ure2p and Sup35p proteins respectively thus presence of the prion form leads to a similar phenotype as genetic inactivation of the corresponding gene. In the case of [Het-s], the prion form is not detected as a loss of function of the corresponding protein. Rather transition to the prion state is detected because the protein gains a novel property (that of triggering cell death when interacting with HET-S). In that the [Het-s] system is more similar to mammalian prions or also to the [PIN+] yeast prion.Citation14
Het-s Aggregation and Generation of [Het-s]-infectivity In Vitro
Using GFP fusion proteins and fractionation techniques it could be shown that the HET-s protein aggregates specifically upon transition to the prion state.Citation15 The protein exists as a soluble monomer in [Het-s*] strains and forms high molecular weight aggregates in [Het-s] strains when highly expressed. Renatured purified recombinant histidine tagged HET-s protein is initially soluble and monomeric and spontaneously undergoes a conformational transition to an aggregated fibrillar form.Citation16 HET-s aggregates are typical amyloids showing a high β-sheet content, proteinase K resistance, and Congo red birefringence.Citation16
The prion hypothesis for [Het-s] could be directly proven by introducing amyloid aggegates of HET-s into a [Het-s*] mycelium.Citation17 This was achieved using a biolistic approach. A [Het-s*] mycelium overlaid with HET-s protein was bombarded with tungsten particles to force the protein into the cells. This procedure induced appearance of the [Het-s] prion state at an elevated frequency (up to 99% efficiency). Neither the soluble form of the protein nor amorphous heat denatured HET-s aggregates induced appearance of [Het-s]. This experiment provided a direct proof of the protein-only hypothesis in the case of [Het-s] and also strongly suggested that amyloid aggregates of HET-s constitute the infectious prion species. It should however be noted that the fact that amyloids of HET-s are infectious does not prove that the entity propagating in vivo is an amyloid form of HET-s.
Structural Characterisation of Het-s
Studies of the [URE3] and [PSI+] yeast prions revealed the existence of so-called prion forming domains (PFD). Prion forming domains are discrete regions of the prion protein both necessary and sufficient for conferring prion behaviour. In the case of yeast prions, these prion forming domains are N-terminal and characterized by a strong bias in amino acid composition as they are very rich in asparagine and glutamine residues.Citation18
When submitted to proteinase K digestion, amyloid fibrils of HET-s display a 7–8 kDa resistant fragment that could be identified as the C-terminal part of the protein ranging from residue 218 to 289.Citation19 Proteinase K treated fibrils retain their fibrillar state (although the fibril width decreases) and retain infectivity.Citation17 These observations suggested that this C-terminal region could constitute the PFD of HET-s. In vitro and in vivo studies confirmed that the 218–289 region is both necessary and sufficient for [Het-s] prion activity.Citation19,Citation20 It could be shown that soluble HET-s displays two distinct domains, a N-terminal a-helical globular domain spanning residue 1 to about 220 and a C-terminal highly flexible tail. It is this flexible tail that undergoes a major conformational transition from a random coil to a β-sheet rich structure upon aggregationCitation19,Citation21 ().
The sequences appended to the prion forming domain strongly influence the type of supramolecular assemblies formed in vivo. In particular, it was found that in constrast to HET-s-GFP and HET-s(218–289)-GFP fusion proteins which form dot-like aggregates in vivo, the HET-s(157–289)-GFP fusion protein (which retains only a small part of the globular domain region) forms elongated aggregates in vivo that can reach up to 150 µ in length.Citation22
A combination of hydrogen/deuterium exchange, solid state NMR and mutational approaches established a structure model for HET-s(218–289) in its amyloid conformation.Citation23 NMR approaches delimited four β-strands (β1–β4). Remarkably, β1 and β3 and β2 and β4 respectively share a sequence similarity suggesting that the HET-s PFD was generated by an ancient duplication event. The proposed model corresponds to a pseudo-dimer structure with a double β-strand-turn-β-strand motif connected by a large loop (). Proline substitutions in any of the four β-strands abolish or strongly reduce infectivity and aggregation indicating that the ability to form the β-fold is critical for infectivity. This correlation between structure and infectivity strongly suggests that the proposed structure corresponds to the infectious fold of the protein. In contrast to many other amyloids, the solid state NMR spectra of HET-s(218–289) amyloids are characterized by extremely narrow peaks which reflects a very high level of structural organisation in the β-strand regions.Citation23,Citation24
An Infectious Protein Encoded by An Invasive Allele
In 1965, Jean Bernet yet another former pupil of Georges Rizet described a remarkable property of [Het-s] in the sexual cycle.Citation25 Georges Rizet had shown that in a sexual cross of a prion infected [Het-s] strain with het-S, the prion is lost in the meiotic progeny. For this reason, he stated that the S factor has the ability to inactivate [Het-s] (again this explains the original designation of [Het-s*]: “sS” that should be understood as “s modified by S”). Now, when a [Het-s] x [Het-S] cross is performed at low temperature (18° instead of the usual 25°) and when [Het-s] is used as maternal parent something remarkable happens.Citation25,Citation26 A proportion of the asci (about 20%) are two spored instead of four spored. In these two spored asci, the remaining two spores are aborted. Bernet found that the two surviving spores are always of the het-s genotype indicating that the killed spores have the het-S genotype. In addition, in two spored asci the het-s spores contain [Het-s] but in four spored asci, the het-s spores produce [Het-s*] mycelia. No two spored asci are observed in a [Het-s*] X [Het-S] cross.
This het-S-spore killing is though to result from the toxic [Het-s]/HET-S interaction. In other words, when the HET-S protein is expressed in the maturating het-S spores, the interaction with the [Het-s] prion originating from the maternal cytoplasm leads to abortion of the het-S spores. Spore killing is asymmetric, in the reciprocal cross with the [Het-s] strain as the paternal parent there is no occurrence of spore killing. The male gametes (termed microconidia) contribute very little if any cytoplasm to the zygote, as a result the prion does not enter the sexual cycle when carried by the paternal parent. The percentage of killing varies during maturation of the fruiting body. Up to 60% of the asci in young perithecia show spore killing while in the older perithecia only 5% of the asci show killing. During the asci formation, the transition from coenocytic to cellular state results in a stochastic distribution of [Het-s] particles. Lowering of killing percentages with perithecial age, can be explained by hypothesizing that the young asci inherit most of the prion aggregates from the maternal cytoplasm. If the het-s expression stops during the sexual cycle, the late asci would inherit fewer prion seeds and thus killing percentages would diminish.Citation27 An alternative hypothesis, is that the progressive build up of the HET-S protein in the ascogonium during perithecial maturation leads to curing of the [Het-s] prion in late asci. Indeed, it is important to note that in this cross, the majority of the het-s ascospores are cured from the prion (even though the maternal parent was [Het-s]), this loss of [Het-s] indicates that het-S exerts a prion curing effect in a large fraction of the asci.
Spore killing efficiency can be highly increased (> 80% killing) and rendered independent of the temperature by overexpressing the het-s gene.Citation26 The HET-s PFD is strictly required for the spore-killing activity.Citation27 The spore-killing activity empowers the het-s allele with the ability to be over-represented over het-S in the progeny of a het-s x het-S cross; het-s has thus the ability to cheat with Mendelian segregation and behaves as a meiotic drive element. Meiotic drive elements are ultra-selfish chromosomal genes that are genetically invasive and can reach high levels in populations without conferring any fitness benefit to the organism that harbors them.Citation28,Citation29 Meiotic drive activity of the het-s allele is a direct consequence of the prion behavior of HET-s. In other words, the het-s allele is invasive because it encodes an infectious protein. Why then are there still het-S alleles around? Why hasn't het-s reach fixation? One can envision a number of reasons for that. First, maybe het-s is on its way to fixation and Rizet arrived just in time to discover [Het-s]. Indeed without het-S alleles, [Het-s] would have never been discovered. Then, het-S might have a cellular function and thus a direct fitness advantage over het-s that would compensate for the meiotic drive effect. Third, if the biological function of the het-s/het-S system is somatic nonself recognition then these alleles are expected to be under balancing selection (i.e., equilibrated frequencies of the two alleles would be selected for thus preventing fixation of either one of them). Finally and maybe most importantly, is the battle occurring in the [Het-s] X het-S outcross, if the het-S side clearly loses on the genetic ground, het-S still has an epigenetic revenge as it leads to inactivation of the prion in most asci. This prion-curing effect of het-S might potentially greatly decrease the efficiency of het-s meiotic drive in natural populations and might represent the only natural situation of prion-curing in the wild.
Concluding Remarks, the Road Ahead…
More than half a century after its discovery, [Het-s] has unveiled part of its mystery. Thanks to the careful pioneering work of Georges Rizet and Janine Beisson and the molecular characterisation of the gene carried out by Joël Bégueret, the prion concept could be applied to this system. It is now clear that the [Het-s] genetic element is a protein. Recent work on [Het-s] and convergent evidence from the yeast prion field strongly suggest that [Het-s] corresponds to an amyloid or amyloid-like β-sheet-rich aggregated form of the HET-s protein. Nevertheless, as for all other prions, the exact nature of the entity propagating in vivo is not really known. Similarly, the mechanism of spontaneous prion appearance is not understood. In addition to these central questions on prion propagation which are common to all systems, [Het-s] also holds a number of more private secrets. Questions regarding the mechanism of incompatibility (why is the HET-S/HET-s interaction toxic?) as well the evolutionary history of het-s remain totally unanswered. The “petit s” element now stands in this chiaroscuro, with part of its mystery in the light and many more puzzles still hidden in the dark.
Figures and Tables
Figure 1 Life cycle of Podospora anserina. Podospora anserina is a coprophilic filamentous ascomycetes. The ascospores are the meiotic progeny and constitute the resistance form. After ascospore germination the fungus grows as a network of interconnected vegetative filaments -hyphae- with incomplete crosswalls. These hyphae can spontaneously fuse. If hyphal fusion involves incompatible strains the mixed fusion cell undergoes cell death. Upon nutrient starvation and exposure to light, the mycelium differentiates both male and female reproductive organs. The male gametes are termed microconidia. The female gamete is a large cell termed ascogonium and is contained in an organ called protoperithecium. After fertilization the male nucleus reaches the ascogonium and male and female nuclei undergo several mitoses. At this stage there is a transition from a coenocytic to a cellular state. A pair of nuclei of opposite mating-type enter a specialized hypha -the ascogenous hypha- and form a structure termed crozier, an isolated binucleate cell is formed in which meiosis takes place. This cycle is repeated over and over again so that a single fertilization event actually leads to about 50 independent meioses. After a post-meiotic mitosis, nuclei are packed into ascospores, each containing two nonbrother nucleic of the same half tetrad. As a result, ascospores will be homokaryotic for markers showing first division segregation but heterokaryotic for markers showing second division segregation (as in the example depicted here). The mating-type locus shows over 98% second division segregation, ascospores thus nearly invariably contain a nucleus of the + mating-type and a nucleus of the - mating type and thus give rise to self-fertile strains. The het-s locus is closely linked to the centromere and thus shows 95% first division segregation, thus in a het-s x het-S cross, asci nearly invariably contain two het-s and two het-S spores. Occasional mispackage events lead to formation of mononucleate ascospores and thus give rise to 5 spored asci. Strains originating from such mononucleate ascospores are single mating-type and thus self-sterile. They are used for genetic analyses. Note that the organism is haploid throughout its life cycle, the only diploid stage is the zygote.
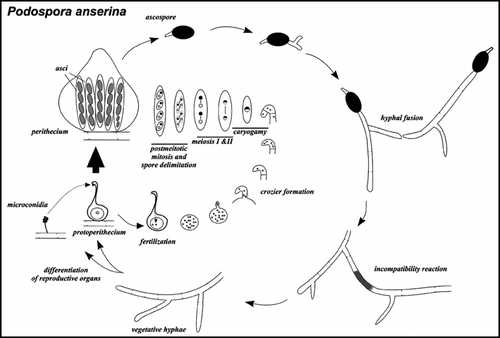
Figure 2 Properties of the s element ([Het-s]) during the sexual cycle. s and S strains are incompatible at the vegetative stage. In a s x S sexual cross (1 and 2), one recovers the expected 50% S progeny but no s progeny are recovered instead a novel phenotype designated sS appears, sS strains are compatible both with s and S. Even when the s parent is used as maternal parent—contributing most of the cytoplasm to the zygote—there are very few s progeny; Janine Beisson reports that in the analysis of 727 asci, only one contained s progeny. In s x sS crosses (3 and 4), all progeny have the phenotype of the maternal parent, which illustrates maternal inheritance of the s element. In a s x S cross performed at low temperature (18°C) when s is used as maternal parent (5), there is a specific abortion of S spores (spore killing), the s gene exerts a meiotic drive effect. In two-spored asci, the two remaining spores display the s phenotype. Color coding is black for s, grey for sS and white for S. (after Rizet, 1952, Beisson-Schecroun 1962, Bernet 1965).
![Figure 2 Properties of the s element ([Het-s]) during the sexual cycle. s and S strains are incompatible at the vegetative stage. In a s x S sexual cross (1 and 2), one recovers the expected 50% S progeny but no s progeny are recovered instead a novel phenotype designated sS appears, sS strains are compatible both with s and S. Even when the s parent is used as maternal parent—contributing most of the cytoplasm to the zygote—there are very few s progeny; Janine Beisson reports that in the analysis of 727 asci, only one contained s progeny. In s x sS crosses (3 and 4), all progeny have the phenotype of the maternal parent, which illustrates maternal inheritance of the s element. In a s x S cross performed at low temperature (18°C) when s is used as maternal parent (5), there is a specific abortion of S spores (spore killing), the s gene exerts a meiotic drive effect. In two-spored asci, the two remaining spores display the s phenotype. Color coding is black for s, grey for sS and white for S. (after Rizet, 1952, Beisson-Schecroun 1962, Bernet 1965).](/cms/asset/cd14c2c0-6a8b-4d7c-95f8-61c4ed6cf4c2/kprn_a_10904666_f0002.gif)
Figure 3 Domain organisation and structural model of HET-s. In (A) a diagram of the HET-s domain organization is given. In its soluble form, the protein displays a N-terminal α-helical globular domain spanning approximately residue 1 to 240 followed by a flexible C-terminal tail. In the amyloid form, the C-terminal region adopts a β-sheet rich conformation composed of four β-strands. The grey shading represents the fibril core. In (B) the sequence of the HET-s PFD is given. The four β-strands are colored. Below an amino acid and nucleic acid alignment of the β1–β2 and β3–β4 regions is given. Numbering correspond to the amino acid position in the HET-s sequence.
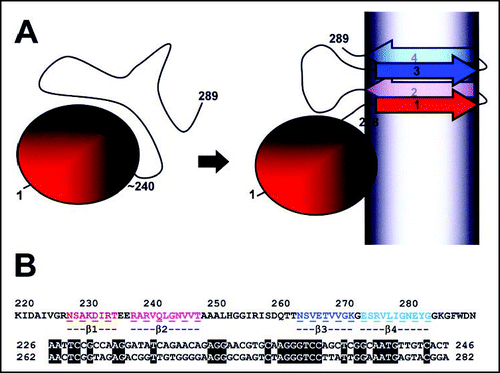
Table 1 [Het-s] nomenclature
Note
There have been some recently added chapters to the [Het-s]-story: it was shown that the prion forming domain of HET-s can propagate as a prion in an heterologous host namely Saccharomyces cerevisiaeCitation30; mass-per-length analysis and electron diffraction studies of HET-s(218–289) fibrils support the current pseudo-dimer model for the amyloid prion form of HET-sCitation31 and finally, it appears that HET-s(218–289) is able to adopt in vitro an alternate amyloid conformation that lacks prion infectivity.Citation32
References
- Rizet G. Les phénomènes de barrage chez Podospora anserina. I. Analyse de barrage entre les souches s et S. Rev Cytol Biol Veg 1952; 13:51 - 92
- Glass NL, Kaneko I. Fatal attraction: Nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot Cell 2003; 2:1 - 8
- Buss LW. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc Natl Acad Sci USA 1982; 79:5337 - 5341
- Saupe SJ. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol Mol Biol Rev 2000; 64:489 - 502
- Beisson-Schecroun J. Incompatibilité cellulaire et interactions nucléocytoplamsiques dans les phénomènes de barrage chez le Podospora anserina. Ann Genet 1962; 4:3 - 50
- Belcour L. Loss of a cytoplasmic determinant through formation of protoplasts in Podospora. Neurospora Newslett 1976; 23:26 - 27
- Turcq B, Denayrolles M, Bégueret J. Isolation of two allelic incompatibility genes s and S of the fungus Podospora anserina. Curr Genet 1990; 17:297 - 303
- Turcq B, Deleu C, Denayrolles M, Bégueret J. Two allelic genes responsible for vegetative incompatibility in the fungus Podospora anserina are not essential for cell viability. Mol Gen Genet 1991; 228:265 - 269
- Deleu C, Clave C, Begueret J. A single amino acid difference is sufficient to elicit vegetative incompatibility in the fungus Podospora anserina. Genetics 1993; 135:45 - 52
- Deleu C, Turcq B, Begueret J. Repa, a repetitive and dispersed DNA sequence of the filamentous fungus Podospora anserina. Nucleic Acids Res 1990; 18:4901 - 4903
- Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci USA 1997; 94:9773 - 9778
- Wickner RB. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae [see comments]. Science 1994; 264:566 - 569
- Satpute-Krishnan P, Serio TR. Prion protein remodelling confers an immediate phenotypic switch. Nature 2005; 437:262 - 265
- Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: The story of [PIN+]. Cell 2001; 106:171 - 182
- Coustou-Linares V, Maddelein ML, Bégueret J, Saupe SJ. In vivo aggregation of the HET-s prion protein of the fungus Podospora anserina. Mol Microbiol 2001; 42:1325 - 1335
- DosReis S, Coulary-Salin B, Forge V, Lascu I, Begueret J, Saupe SJ. The HET-s prion protein of the filamentous fungus Podospora anserina aggregates in vitro into amyloid-like fibrils. J Biol Chem 2002; 277:5703 - 5706
- Maddelein ML, DosReis S, Duvezin-Caubet S, Coulary-Salin B, Saupe SJ. Amyloid aggregates of the HET-s prion protein are infectious. Proc Natl Acad Sci USA 2002; 99:7402 - 7407
- Wickner RB, Taylor KL, Edskes HK, Maddelein ML. Prions: Portable prion domains. Curr Biol 2000; 10:R335 - R337
- Balguerie A, DosReis S, Ritter C, Chaignepain S, Coulary-Salin B, Forge V, Bathany K, Lascu I, Schmitter JM, Riek R, Saupe SJ. Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. EMBO J 2003; 22:2071 - 2081
- Nazabal A, Maddelein ML, Bonneu M, Saupe SJ, Schmitter JM. Probing the structure of the infectious amyloid form of the prion forming domain of HET-s using high-resolution hydrogen/deuterium exchange monitored by mass spectrometry. J Biol Chem 2005;
- Nazabal A, DosReis S, Bonneu M, Saupe SJ, Schmitter JM. Conformational transition occurring upon amyloid aggregation of the HET-s prion protein of Podospora anserina analyzed by hydrogen/deuterium exchange and mass spectrometry. Biochemistry 2003; 42:8852 - 8861
- Balguerie A, DosReis S, Coulary-Salin B, Chaignepain S, Sabourin M, Schmitter JM, Saupe SJ. The sequences appended to the amyloid core region of the HET-s prion protein determine higher-order aggregate organization in vivo. J Cell Sci 2004; 117:2599 - 2610
- Ritter C, Maddelein ML, Siemer AB, Lührs T, Ernst M, Meier BH, Saupe SJ, Riek R. Correlation of structural elements and infectivity of the HET-s prion. Nature 2005; 435:844 - 848
- Siemer AB, Ritter C, Ernst M, Riek R, Meier BH. High-resolution solid-state NMR spectroscopy of the prion protein HET-s in its amyloid conformation. Angew Chem Int Ed Engl 2005; 44:2441 - 2444
- Bernet J. Mode d'action des gènes de barrage et relation entre l'incompatibilité cellulaire et l'incompatibilité sexuelle chez le Podospora anserina. Ann Sci Natl Bot 1965; 6:611 - 768
- Dalstra HJ, Swart K, Debets AJ, Saupe SJ, Hoekstra RF. Sexual transmission of the [Het-S] prion leads to meiotic drive in Podospora anserina. Proc Natl Acad Sci USA 2003; 100:6616 - 6621
- Dalstra HJ, vanderZee R, Swart K, Hoekstra RF, Saupe SJ, Debets AJ. Nonmendelian inheritance of the HET-s prion or HET-s prion domains determines the het-S spore killing system in Podospora anserina. Fungal Genet Biol 2005; 42:836 - 847
- Lyttle TW. Cheaters sometimes prosper: Distortion of mendelian segregation by meiotic drive. Trends Genet 1993; 9:205 - 210
- Pennisi E. Meiotic drive: Bickering genes shape evolution. Science 2003; 301:1837 - 1839
- Taneja V, Maddelein ML, Talarek N, Saupe SJ, Liebman SW. A non-Q/N-rich prion domain of a foreign prion, [Het-s], can propagate as a prion in yeast. Mol Cell 2007; 27:67 - 77
- Sen A, Baxa U, Simon MN, Wall JS, Sabate R, Saupe SJ, Steven AC. Mass analysis by scanning transmission electron microscopy and electron diffraction validate predictions of stacked β-solenoid model of HET-s prion fibrils. J Biol Chem 2007; 282:5545 - 5550
- Sabate R, Baxa U, Benkemoun L, SanchezdeGroot N, Coulary-Salin B, Maddelein ML, Malato L, Ventura S, Steven AC, Saupe SJ. Prion and Non-prion Amyloids of the HET-s Prion forming Domain. J Mol Biol 2007; 370:768 - 783