Abstract
We identified the 37/67kDa laminin receptor (LRP/LR) as a cell surface receptor for the cellular prion protein (PrPc) and the infectious prion protein (PrPSc). Recently, we showed that anti-LRP/LR antibody W3 cured scrapie infected N2a cells. Here, we demonstrate that W3 delivered by passive immunotransfer into C57BL/6 mice reduced the PrPSc content in the spleen significantly by 66%, demonstrating an impairment of the peripheral PrPSc propagation. In addition, we observed a 1.8 fold increase in survival of anti-LRP/LR antibody W3 treated mice (mean survival of 31 days) compared to preimmune serum treated control animals (mean survival of 17 days). We conclude that the significant effect of anti-LRP/LR antibody W3 on the reduction of peripheral PrPSc propagation might be due to the blockage of the prion receptor LRP/LR which is required, as previously shown in vitro, for PrPSc propagation in vivo.
Introduction
Transmissible spongiform encephalopathies (TSE) are a group of neurodegenerative protein-misfolding diseases, also known as prion diseases affecting both animals including scrapie in sheep, bovine spongiform encephalopathy (BSE) in cattle and chronic wasting disease (CWD) in elk and deer as well as humans (e.g., Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker (GSS) syndrome and fatal familial insomnia (FFI)) (for review see refs. Citation1–Citation4). Affected individuals display rapidly progressive symptoms due to various effects such as gliosis, astrocytosis, neuronal loss and spongiosis.
TSEs are associated with an abnormal form of the prion protein, termed PrPSc. The conversion of the host encoded PrPc into the disease associated isoform (PrPSc) results in accumulation which is perpetuated by an autocatalytic process.Citation5,Citation6 Although various therapeutic approaches have already been developed (for review see refs. Citation7–Citation11), no treatment was available until now, which is able to cure affected individuals. The first successful approach in antibody-based therapies was the passive immunotransfer of a monoclonal anti-PrP antibody which cured rodents peripherally infected with PrPSc.Citation12 Besides monoclonal antibodies targeting the prion protein,Citation13,Citation14 also single chain anti-PrP antibodies are currently investigated for a TSE therapy.Citation15
Among many interaction partners identified for PrPc,Citation10,Citation16–Citation18 the non-integrin 37/67 kDa laminin receptor (LRP/LR) has been discovered as a receptor for both the cellular PrPc Citation19,Citation20 and the disease associated PrPSc.Citation21,Citation22 Downregulation of LRP/LR by antisense LRP RNA or siRNAs directed against LRP mRNA abrogates PrPSc propagation in ScN2a cells.Citation23 Secretion of a transdominant negative LRP mutant also abolishes PrPSc propagtion in neuronal cells.Citation24 A polyclonal anti-LRP/LR antibody termed W3 interferes with PrP27–30 cell bindingCitation21 and internalization of bovine PrPSc by human enterocytes.Citation22 Most notably, W3 has been shown to cure PrPSc propagating cells from scrapie.Citation23 In order to investigate whether W3 is able to hamper prion propagation in vivo, we delivered W3 into mice by passive immunization. Spleen analysis confirmed a significant reduction in peripheral PrPSc propagation in W3 treated mice. Moreover, W3 treated mice revealed a 1.8-fold increase in survival (the time span from the day one of the four TSE-relevant symptoms occur until the day mice show two of the four TSE-relevant symptoms over three daysCitation25) compared to the control group injected with preimmune serum. Our results suggest that LRP/LR plays an important role for PrPSc propagation in vivo and that targeting LRP/LR is a relevant strategy for therapy in prion diseases.
Materials and Methods
Antibodies and preimmune serum.
In order to get the polyclonal anti-LRP antibody pAb W3, we immunized albino rabbits [(New Zealand; ZRL:kbl (nzw)br; Charles River Breeding Laboratories, Wilmington, Massachusets)]. One mililiter of a mixture of GST:: LRP fusion protein expressed in E. coli systemCitation26 and CWS adjuvant (RIBI adjuvant, Sigma) was subcutaneously injected into rabbits (see ref. Citation27). After 28 days, animals were boosted and after additional 14 days the animals were immunized a third time. Eleven days later 200 ml of blood were collected and coagulated for one hour at 37°C and incubated over night at 4°C followed by two centrifugation steps ten minutes at 9,000 rpm and 10.500 rpm at 4°C. Purification was done using a protein A sepharose column (Pierce, Rockford, Illinois). W3 was selected from several anti-LRP sera tested for recognition efficiency of LRP/LR by FACS and western analysis.Citation27 Preimmune serum was obtained from rabbit prior to immunization.
Passive immunotransfer of anti-LRP/LR antibody W3 into mice.
Animals were maintained and treated in accordance with ethical guidelines of Bavaria. Experiments were approved by the Regierung von Oberbayern (Munich, Germany, Ar.: 209.1/211-2531-83/04). For infection studies C57BL/6 mice were injected intraperitoneally (i.p.) with a total amount of 1 mg of W3 or preimmune serum. Treatment was performed once per week over a period of 12 weeks. One week after the first antibody injection mice were inoculated i.p. with 100 µl 10% RML Scrapie homogenate. The time span from the day of RML inoculation until one of the four symptoms: ataxia of gait, tremor, difficulty righting from a supine position and rigidity in the tail occured (termed as symptom onset) and survival (the time span from the day one of the four TSE-relevant symptoms occur until the day mice show two of the four TSE-relevant symptoms over three daysCitation25) were monitored. In all monitoring procedures the investigators were blinded as to the experimental groups individual mice belonged to.
Analysis of PrPSc and total PrP levels in the spleen of RML inoculated mice.
Ninety days post RML inoculation six mice per group were sacrificed for analysis of peripheral PrPSc propagation. Spleens were collected and homogenized in PBS buffer. Adjusting the total protein amount to 200 µg, samples were digested with Proteinase K to a final concentration of 20 µg/ml for 60 minutes at 37°C. Samples were analysed on a 12% SDS PAGE and blotted onto a PVDF membrane. Immunodetection was performed using SAF83 as the primary and anti-mouse-POD conjugate (Jackson Immunoresearch) as the secondary antibody. Blots were developed using an enhanced chemiluminescence system (Perkin Elmer Lifescience) and exposed on Kodak Biomax light films. Quantification of the western blot signals was carried out by densitometric measurements using the Image J software. To determine the total PrP amount, spleen samples were treated as described for the PrPSc detection but without a Proteinase K treatment. For total PrP detection SAF32 was used as the primary and anti-mouse-IgG-POD as the secondary antibody.
Analysis of PrPSc and total PrP levels in the brain of terminal mice.
Mice were sacrified after two of the four characteristical TSE symptomsCitation25 were detected for a period of three days. Total brain samples of six mice per group were collected and homogenized in PBS buffer. Protein levels were adjusted to 200 µg per sample and digested with Proteinase K to a final concentration of 20 µg/ml for 60 minutes at 37°C. The PrPSc content was determined by analysis on a 12% SDS PAGE and blotted onto a PVDF membrane. Immunodetection was performed using SAF83 as the primary and anti-mouse-IgG-POD (Jackson Immunoresearch) as the secondary antibody. Blots were developed using an enhanced chemiluminescence system (Perkin Elmer Lifescience) and exposed on Kodak Biomax light films. Quantification of the western blot signals was carried out by densitometric measurements using the Image J software. To determine the total PrP amount total brain samples were treated as described for PrPSc detection in the absence of Proteinase K treatment. Detection for total PrP was carried out using SAF32 as the primary and anti-mouse-IgG-POD as the secondary antibody.
Statistical analysis.
Statistical analyses were performed employing a Student's t test with two tailed distribution and two-sample unequal variance.
Results
Anti-LRP/LR antibody W3 reduces peripheral PrPSc propagation.
One milligram per week of anti-LRP/LR antibody W3 and preimmune serum was intraperitoneally injected into scrapie infected mice for a period of 12 weeks (). One week after the first injection mice were intraperitoneally inoculated with RML Scrapie homogenate and monitored for symptom onset (incubation times) and survival (). W3 and preimmune serum treated mice were controlled daily and showed normal behavior till they were sacrificed. No side effects were detectable at any time points.
Western blot analysis of the spleen of W3 treated mice 90 days post inoculation revealed a significant reduction of the PrPSc level by 66% compared to the preimmune serum treated mice ( and ), suggesting that W3 reduces significantly peripheral PrPSc propagation. The amount of total PrP (PrPSc plus PrPc) was reduced by approx. 40% ( and ) in W3 treated mice, suggesting that PrPc levels remain unaffected by the antibody treatment.
Anti-LRP/LR antibody W3 prolongs the survival in scrapie infected mice.
Anti-LRP/LR antibody W3 treated mice did not show a prolongation of incubation times compared to the preimmune serum treated control group ( and ). However, the anti-LRP/LR antibody W3 treated mice revealed a mean survival of 31 days, which represents a 1.8-fold prolongation of the survival in comparison with the preimmune serum (mean survival 17 days) treated control group (, and ).
At the terminal state the PrPSc level in the brain of W3 treated mice was reduced by 17% compared to preimmune serum treated mice ( and ), whereas W3 and preimmune serum treated mice showed no alteration in the total PrP content ( and ), suggesting that PrPc levels remained unaltered in both experimental groups.
Discussion
At present no therapeutic strategy is available for the treatment of TSEs which cures prion diseases.Citation8,Citation9,Citation11,Citation28,Citation29 Since the laminin receptor acts as a receptor for PrPc,Citation30 and PrPSc,Citation21 LRP/LR exhibits a promising target for therapeutic strategies in prion diseases. The anti-laminin receptor polyclonal antibody W3 was well efficient in vitro by curing scrapie propagating cells from PrPSc.Citation23 In order to prove an in vivo effect of W3 on (1) peripheral PrPSc propagation and (2) prolongation of survival, we passively transferred W3 into C57BL/6 mice by intraperitoneal injections followed by i.p. RML prion inoculations. W3 treated mice revealed a significant reduction (66%) of the peripheral PrPSc propagation compared to preimmune serum treated mice as analyzed by determining the PrPSc levels in the spleen. Total PrP levels (PrPSc plus PrPc) in the spleen were reduced by approx. 40%, suggesting that W3 treatment has no or only a weak influence on the reduction of PrPc levels in the spleen.
In addition, W3 treated mice revealed a 1.8-fold increase in survival (31 days) compared to the preimmune serum treated control group (17 days), suggesting that W3 also hampers PrPSc propagation in the central nervous system contributing to prolongation of survival. Moreover, this is of potential interest for treatment of CJD patients because the effects were observed during the clinical stages, when mice had already neurological symptoms (prolongation of survival time from onset of the disease to death). Due to the limited amount of the polyclonal antibody W3, which was raised against LRP in a rabbit,Citation27 we could not elongate the antibody treatment over the 12 weeks treatment period and could not increase the number of animals and/or the dose of antibody applied (1 mg) which all together might have resulted in an even more obvious effect on the increase in survival. In contrast to survival, incubation times were not affected. We assume that by increasing the antibody amount and the time period of treatment, the incubation time might also be prolonged. Application of a monoclonal anti-PrP antibody in mice with 2 mg of antibody twice a week resulted in a delayed onset of the disease.Citation12 Many promising anti-prion drugs which are effective in vitro failed to be active also in vivo (reviewed in ref. Citation9). Among them, are the antimalaria drugs mefloquineCitation31 and quinacrine.Citation32 In contrast, W3 is effective both in vitroCitation23 and in vivo. Currently, the efficacy of doxycyclin in the treatment of CJD is under investigation in observational studies in Milano (Italy) and Göttingen (Germany). First data indicate that administration of doxycyclin might prolong the survival by two-fold. In the German study on 23 patients, the survival was prolonged from four months (median in sCJD) to eight months. Whether the prolongation time is due to a specific prion effect, will be tested in a prospective double blind study. No conclusions can be drawn, however, regarding a potential prolongation of incubation times in CJD patients by doxycyclin. A combination therapy with doxycyclin and antibodies targeting LRP/LR might have some additive or even synergistic effects.
On the molecular level LRP/LR specific antibody W3 blocks (1) PrPc binding to neuronal cells,Citation30 (2) PrP27-30 binding to mammalian cellsCitation21 and (3) BSE prion internalization by human enterocytes,Citation22 suggesting that the antibody interferes with PrPc and PrPSc internalization processes prohibiting as a consequence PrPSc propagation, which might occur in compartments of the endocytic pathway rather than on the cell surface (for review see refs. Citation8 and Citation10). In scrapie infected mice, we show that W3 is able to interfere efficiently with peripheral PrPSc propagation, which takes place in organs of the lymphoreticular system such as the spleen. At the time point when the animals were sacrificed (at the day when two TSE-associated symptoms appeared for three daysCitation25) the detected PrPSc levels in the brain were not significantly different between W3 treated and preimmune serum-treated mice, suggesting that PrPSc propagation was not delayed in the brain of W3 treated animals. The total PrP content at the terminal state in the brain of mice treated with W3 and preimmune serum was unchanged, suggesting that also PrPc levels in the brain were not affected by W3 treatment.
Taken together, this pilot study revealed important results regarding an antibody therapy or post-exposure prophylaxis with W3 resulting in a signifcant reduction of peripheral PrPSc propagation and a slight prolongation of survival. Since we started the antibody treatment seven days prior to PrPSc inoculation and terminated the treatment 77 days post PrPSc inoculation (long before first symptoms occur) (), we preformed in this study a post-exposure prophylaxis rather than a therapy, which is usually initiated at the stage when first symptoms appeared.
Since the amount of W3 is limited, we generated in a parallel study single chain antibodies (scFv) directed against LRP/LR by phage display.Citation33 Passive immunotransfer of the scFv S18 by intraperitoneal injections into scrapie infected mice also resulted in a reduction of the PrPSc level in the spleen by approx. 40% without a significant prolongation of incubation times and survival.33 W3 reduced PrPSc levels in the spleen significantly by 66% and slightly prolonged the survival 1.8-fold. One of the reasons for the slightly better efficacy of W3 compared to scFv S18 might be the longer half-life of full-length IgGs in the blood (approximately 14 days) compared to single chain antibodies (approximately 12 hours). The amount of antibody (1 mg per week) was the same for both studies although the duration of antibody treatment was different (eight weeks for scFv S18 versus 12 weeks for W3). A polyclonal serum raised against a specific antigen (here LRP/LR) contains approximately 5–10% antibodies directed against this antigen.Citation34 Therefore approximately 50–100 mg of LRP/LR specific antibodies per week were injected in the present study, i.e. 10 to 20-fold less compared to the scFv S18 trial (1 mg/week), suggesting that full-length IgG molecules are more potent than scFv fragments in passive immunotherapy.
Our results demonstrated that the polyclonal antibody W3 significantly impaired PrPSc replication in the spleen. These findings are an incentive to pursue with studies of antibodies directed against LRP/LR to obtain more effective results on the neuroinvasion phases of the infection. Passive immunotransfer studies with improved versions of the single chain antibody S18, as well as full-length IgG versions thereof might represent promising regimens for an efficient treatment of prion diseases.
Abbreviations
| CJD | = | Creutzfeldt-Jakob disease |
| CWD | = | chronic wasting disease |
| FFI | = | fatal familial insomnia |
| GSS | = | Gerstmann-Sträussler-Scheinker |
| LR | = | high affinity laminin receptor |
| LRP | = | laminin receptor precursor |
| RML | = | rocky mountain laboratory |
| TSE | = | transmissible spongiform encephalopathy |
| W3 | = | polyclonal anti-LRP/LR antibody |
Figures and Tables
Figure 1 Schematic overview of the passive immunotransfer modalities. One milligram of antibody (either W3 or preimmune serum) per C57BL/6 mouse was intraperitoneally injected seven days prior to intraperitoneal inoculation with 10% RML brain homogenate. Antibodies injections (i.p.) were performed at doses of 1 mg per week for a period of 11 weeks. Treatment was terminated 77 days post inoculation. Ninety days post inoculation, animals were sacrificed for analysis of the PrPSc and total PrP content of the spleen. Incubation times represent the time span from the day of RML inoculation until one of the four symptoms occur: ataxia of gait, tremor, difficulty righting from a supine position and occurrence of rigidity in the tail. Survival represent the time span from the day one of the four TSE-relevant symptoms occurs until the day mice show two of the four TSE-relevant symptoms over three days.Citation25 At this time point mice were sacrificed followed by determination of the PrPSc and total PrP content in the brain.
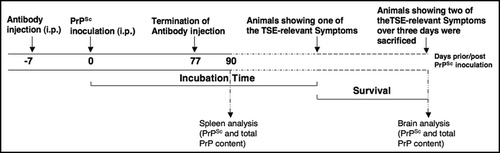
Figure 2 Analysis of total PrP and PrPSc levels in the spleen of scrapie infected mice treated with polyclonal anti-LRP antibody W3 and preimmune serum 90 days post infection. LRP/LR antibody W3 and preimmune serum was intraperitoneally injected into C57BL/6 mice for 12 weeks. C57BL/6 mice were intraperitoneally inoculated with RML prions (10%) one week after the first antibody/preimmune serum injection. (A) Spleen of C57BL/6 mice were collected 90 days post infection and analyzed for the PrPSc content after Proteinase K digestion by Western blotting using anti-PrP antibody SAF 83. Western blot analysis of the PrPSc levels in the spleen of three W3 and three preimmune serum treated mice are shown. β-actin was used as a loading control (detection by an anti-β-actin antibody). (B) Densitometric measurements of Western blots from six spleens per group revealed a significant reduction of the PrPSc level by 66% in the W3 treated group compared to the preimmune serum treated group, for which the PrPSc level was set to 100% (*p < 0.05). Quantification of PrPSc signals were normalized by β-actin levels. Quantification of the western blot signals was carried out by densitometric measurements using the Image J software (mean + SD). (C) Spleen samples of C57BL/6 mice (collected 90 days post infection) were analyzed for quantification of the total PrP content by western analysis using anti-PrP antibody SAF 32. Western blot analysis of the PrP levels (in the absence of Proteinase K) in the spleen of three W3 and three preimmune serum treated mice is shown. β-actin was used as a loading control (detection by an anti-β-actin antibody). (D) Densitometric measurements of western blots from six spleens per group revealed a reduction of total PrP content by 39% in the W3 treated group compared to the preimmune serum treated group, for which the total PrP level was set to 100% (p < 0.2). Quantification of total PrP signals were normalized by β-actin levels. Quantification of the western blot signals was carried out by densitometric measurements using the Image J software (mean + SD).
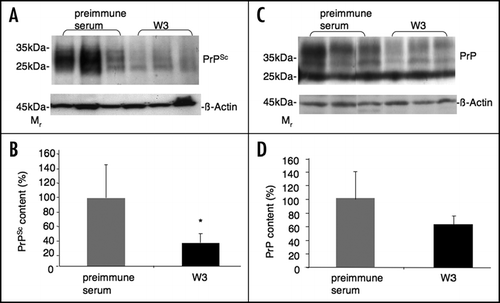
Figure 3 Symptom onset (Incubation times) and survival in scrapie infected C57BL/6 mice treated with polyclonal anti-LRP antibody W3 and preimmune serum. C57BL/6 mice were intraperitoneally inoculated with RML prions one week after the first antibody/preimmune serum injection and monitored for the occurrence of characteristic TSE symptoms. Symptom onset (Incubation times) represents the time span from the day of RML inoculation until the day one of the four symptoms appear: ataxia of gait, tremor, difficulty righting from a supine position and occurance of rigidity in the tail.Citation25 Survival represents the time span from the day one of the four symptoms occurs until the day mice show two of the four TSE-relevant symptoms over three days.Citation25 At this time point, mice were sacrificed. (A) Kaplan-Meier curve (symptom onset) showing percent of animals free of symptoms dependent from days post RML inoculation. 20% of the W3 treated animals revealed a prolonged symptom onset compared to the preimmune serum control group (p < 0.2). (B) Kaplan-Meier curve (symptom onset plus survival) showing percent of animals alive dependent from days post RML inoculation. (C) Survival (days) of W3 and preimmune serum treated animals. The median revealed a 1.8-fold prolonged survival for the W3 treated group compared to the control group injected with preimmune serum (p < 0.19) (mean + SD).
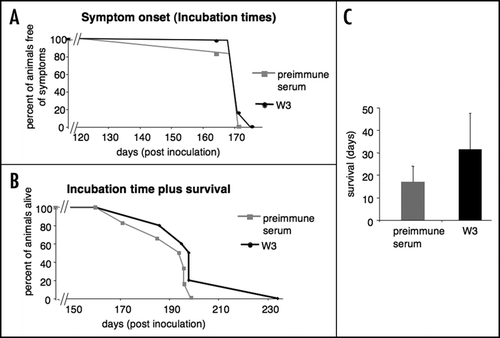
Figure 4 Analysis of total PrP and PrPSc levels in the brain of scrapie infected mice treated with polyclonal anti-LRP antibody W3 and preimmune serum at the terminal state. LRP/LR antibody W3 and preimmune serum was intraperitoneally injected into C57BL/6 mice for 12 weeks. One week after the first antibody/preimmune serum injection C57BL/6 mice were intraperitoneally inoculated with RML prions (10%). (A) Mice were sacrificed after showing two of the four characteristical TSE symptomsCitation25 over three days (terminal state) and total brain was analyzed for the PrPSc content (after Proteinase K digestion) by Western blot analysis using anti-PrP antibody SAF 83. PrPSc levels in total brain samples of three W3 and three preimmune serum treated mice are shown. β-actin was used as a loading control (detection by an anti-β-actin antibody). (B) Densitometric measurements of Western blots from six brain samples per group revealed a reduction of PrPSc levels by 17% in the W3 treated group compared to the preimmune serum treated group, for which the PrPSc level was set to 100% (p < 0.3). Quantification of PrPSc signals were normalized by β-actin levels. Quantification of the Western blot signals was carried out by densitometric measurements using the Image J software (mean + SD). (C) Total brain samples of terminal C57BL/6 scrapie infected mice were analysed for the total PrP content (in the absence of Proteinase K) by Western blot analysis using anti-PrP antibody SAF 32. Total PrP levels in total brain samples of three W3 and three preimmune serum treated mice are shown. β-actin was used as a loading control (detection by an anti-β-actin antibody). (D) Densitometric measurements of western blots from six brains per group revealed approx. equal levels in both groups (p < 0.2). Quantification of total PrP signals were normalized by β-actin levels. Quantification of the Western blot signals was carried out by densitometric measurements using the Image J software (mean + SD).
Table 1 Incubation times and survival of scrapie infected mice treated with W3 and preimmune serum
Acknowledgements
We thank Katharina Krüger, Tina Hallas and Jennifer Hentrich for excellent technical assistance. This work was supported by the Bundesministerium für Bildung und Forschung (grant 01-KO-0514), the European Commission (grant NoE-NeuroPrion FOOD-CT-2004-506579) and the Deutsche Forschungsgemeinschaft (DFG) (grant WE 2664/2-1).
References
- Aguzzi A, Weissmann C. Prion diseases. Haemophilia 1998; 4:619 - 627
- Prusiner SB. Prions. Proc Natl Acad Sci USA 1998; 95:13363 - 13383
- Lasmézas CI, Weiss S. Cary JW, Linz JE, Bhatnagar D. Molecular Biology of Prion Diseases. Microbial Foodborne Diseases Mechanisms of Pathogenicity and Toxin Synthesis 2000; Lancaster (USA) Technomic Publishing CO., INC 495 - 537
- Weissmann C. The state of the prion. Nat Rev Microbiol 2004; 2:861 - 871
- Bieschke J, Weber P, Sarafoff N, Beekes M, Giese A, Kretzschmar H. Autocatalytic self-propagation of misfolded prion protein. Proc Natl Acad Sci USA 2004; 101:12207 - 12211
- Weissmann C. Birth of a prion: Spontaneous generation revisited. Cell 2005; 122:165 - 168
- Aguzzi A, Heikenwalder M, Miele G. Progress and problems in the biology, diagnostics, and therapeutics of prion diseases. J Clin Invest 2004; 114:153 - 160
- Ludewigs H, Zuber C, Vana K, Nikles D, Zerr I, Weiss S. Therapeutic approaches for prion disorders. Expert Rev Anti Infect Ther 2007; 5:613 - 630
- Trevitt CR, Collinge J. A systematic review of prion therapeutics in experimental models. Brain 2006; 129:2241 - 2265
- Vana K, Zuber C, Nikles D, Weiss S. Novel aspects of prions, their receptor molecules, and innovative approaches for TSE therapy. Cell Mol Neurobiol 2007; 27:107 - 128
- Weissmann C, Aguzzi A. Approaches to therapy of prion diseases. Annu Rev Med 2005; 56:321 - 344
- White AR, Enever P, Tayebi M, Mushens R, Linehan J, Brandner S, Anstee D, Collinge J, Hawke S. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 2003; 422:80 - 83
- Luginbuhl B, Kanyo Z, Jones RM, Fletterick RJ, Prusiner SB, Cohen FE, Williamson RA, Burton DR, Pluckthun A. Directed evolution of an anti-prion protein scFv fragment to an affinity of 1 pM and its structural interpretation. J Mol Biol 2006; 363:75 - 97
- Padiolleau-Lefevre S, Alexandrenne C, Dkhissi F, Clement G, Essono S, Blache C, Couraud JY, Wijkhuisen A, Boquet D. Expression and detection strategies for an scFv fragment retaining the same high affinity than Fab and whole antibody: Implications for therapeutic use in prion diseases. Mol Immunol 2007; 44:1898 - 1906
- Pankiewicz J, Prelli F, Sy MS, Kascsak RJ, Kascsak RB, Spinner DS, Carp RI, Meeker HC, Sadowski M, Wisniewski T. Clearance and prevention of prion infection in cell culture by anti-PrP antibodies. Eur J Neurosci 2006; 23:2635 - 2647
- Petrakis S, Sklaviadis T. Identification of proteins with high affinity for refolded and native PrP(C). Proteomics 2006;
- Fasano C, Campana V, Zurzolo C. Prions: Protein only or something more? Overview of potential prion cofactors. J Mol Neurosci 2006; 29:195 - 214
- Lee KS, Linden R, Prado MA, Brentani RR, Martins VR. Towards cellular receptors for prions. Rev Med Virol 2003; 13:399 - 408
- Gauczynski S, Peyrin JM, Haik S, Leucht C, Hundt C, Rieger R, Krasemann S, Deslys JP, Dormont D, Lasmezas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. Embo J 2001; 20:5863 - 5875
- Hundt C, Peyrin JM, Haik S, Gauczynski S, Leucht C, Rieger R, Riley ML, Deslys JP, Dormont D, Lasmezas CI, Weiss S. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. Embo J 2001; 20:5876 - 5886
- Gauczynski S, Nikles D, El-Gogo S, Papy-Garcia D, Rey C, Alban S, Barritault D, Lasmezas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J Infect Dis 2006; 194:702 - 709
- Morel E, Andrieu T, Casagrande F, Gauczynski S, Weiss S, Grassi J, Rousset M, Dormont D, Chambaz J. Bovine prion is endocytosed by human enterocytes via the 37 kDa/67 kDa laminin receptor. Am J Pathol 2005; 167:1033 - 1042
- Leucht C, Simoneau S, Rey C, Vana K, Rieger R, Lasmezas CI, Weiss S. The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO Rep 2003; 4:290 - 295
- Vana K, Weiss S. A Trans-dominant negative 37kDa/67kDa laminin receptor mutant impairs PrP(Sc) propagation in scrapie-infected neuronal cells. J Mol Biol 2006; 358:57 - 66
- Sethi S, Lipford G, Wagner H, Kretzschmar H. Postexposure prophylaxis against prion disease with a stimulator of innate immunity. Lancet 2002; 360:229 - 230
- Weiss S, Famulok M, Edenhofer F, Wang YH, Jones IM, Groschup M, Winnacker EL. Overexpression of active Syrian golden hamster prion protein PrPc as a glutathione S-transferase fusion in heterologous systems. J Virol 1995; 69:4776 - 4783
- Rieger R, Edenhofer F, Lasmezas CI, Weiss S. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med 1997; 3:1383 - 1388
- Rossi G, Salmona M, Forloni G, Bugiani O, Tagliavini F. Therapeutic approaches to prion diseases. Clin Lab Med 2003; 23:187 - 208
- Zuber C, Ludewigs H, Weiss S. Therapeutic approaches targeting the prion receptor LRP/LR. Vet Microbiol 2007; 123:387 - 393
- Gauczynski S, Hundt C, Leucht C, Weiss S. Interaction of prion proteins with cell surface receptors, molecular chaperones, and other molecules. Adv Protein Chem 2001; 57:229 - 272
- Kocisko DA, Caughey B. Mefloquine, an antimalaria drug with antiprion activity in vitro, lacks activity in vivo. J Virol 2006; 80:1044 - 1046
- Barret A, Tagliavini F, Forloni G, Bate C, Salmona M, Colombo L, De Luigi A, Limido L, Suardi S, Rossi G, Auvre F, Adjou KT, Sales N, Williams A, Lasmezas C, Deslys JP. Evaluation of quinacrine treatment for prion diseases. J Virol 2003; 77:8462 - 8469
- Zuber C, Knackmuss S, Rey C, Reusch U, Rottgen P, Frohlich T, Arnold GJ, Pace C, Mitteregger G, Kretzschmar HA, Little M, Weiss S. Single chain Fv antibodies directed against the 37kDa/67kDa laminin receptor as therapeutic tools in prion diseases. Mol Immunol 2008; 45:144 - 151
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. Ilar J 2005; 46:258 - 268