Abstract
This review will first recall the phenomena of “cortical inheritance” observed and genetically demonstrated in Paramecium 40 years ago, and later in other ciliates (Tetrahymena, Oxytricha, Paraurostyla), and will analyze the deduced concept of “cytotaxis” or “structural memory”. The significance of these phenomena, all related (but not strictly restricted ) to the properties of ciliary basal bodies and their mode of duplication, will be interpreted in the light of present knowledge on the mechanism and control of basal body/centriole duplication. Then other phenomena described in a variety of organisms will be analyzed or mentioned which show the relevance of the concept of cytotaxis or structural memory to other cellular processes, mainly (1) cytoskeleton assembly and organization with examples on ciliates, trypanosome, mammalian cells and plants, and (2) transmission of polarities with examples on yeast, trypanosome and metazoa. Finally, I will discuss some aspects of this particular type of non DNA inheritance: (1) why so few documented examples if structural memory is a basic parameter in cell heredity, and (2) how are these phenomena (which all rely on protein/protein interactions, and imply a formatting role of preexisting proteinic complexes on neo formed proteins and their assembly) related to prions?
Introduction
Our understanding of biological processes is constrained within a circular relationship resembling the “hen-egg dilemma” which might be called the “DNA-cell dilemma”: if all the information necessary to make a cell (or a tyrannosaurus, as the fiction goes) is stored in DNA sequences, why does a cell only arise from a preexisting cell? The uninterrupted cell continuity since LUCA suggests that cell heredity might require more than DNA. Aside from membranes, which, like DNA, cannot form de novo, what does a cell transmit to its daughters that allow them to recapitulate the exact morphology of their mother, despite the profound remodeling that accompanies division?
Such a question may come to the mind of any biologist watching his/her favorite cell dividing; however, some cell types are undoubtedly more suggestive of the existence of a cellular memory than others, and it may not be fortuitous that a ciliate, Paramecium, was the first organism to inspire a genetic approach of the problem.Citation1 Ciliates are unicellular organisms characterized by the elaborate asymmetrical organization of their surface (cortex), with the equivalent of multiple different organs, arranged in a specific body plan, so that each division involves complex morphogenetic movements akin to developmental processes. The high fidelity of reproduction of every minute detail of this complex pattern raised two types of questions: Is cell organization genetically determined in all details? Can morphogenetic processes rely only on the specificity of proteins and self-assembly mechanisms or do they also involve the persistence of structural or biochemical landmarks to channel or template assembly?
In the early sixties, T.M. Sonneborn, the American biologist who developed Paramecium as a model genetic system, addressed these questions; he studied different spontaneous or experimental variations in Paramecium cortical organization and demonstrated their hereditary maintenance by cellular continuity, without any change in the DNA-encoded information. This cortical inheritance led to the concept of “cytotaxis,” or “structural inheritance” at about the same time as the non-DNA-based nature of the scrapie agent first began to be suspected.Citation2
The experimental bases of this concept will be reviewed, and its general significance and biological importance as a manifestation of non-DNA-based inheritance will be discussed.
Paramecium: A Model for a New Concept
depicts the organization of the cortex of P. tetraurelia featuring over 4,000 ciliary basal bodies. A ciliate can best be thought of as a metazoan embryo which missed cellularization, but has nevertheless partitioned its surface into repeated units, the cortical units, each with one or two basal bodies flanked by cytoskeletal appendages; the whole forms a mosaic of territories endowed with distinct morphogenetic capacities.Citation3 In addition, diverse organelles fulfill specialized functions, e.g., a ciliated oral apparatus (oa) for phagocytosis, a contractile cytoproct (cy) for excretion of food vacuole residues, a complex vesicular and microtubular system for osmoregulation opening at contractile vacuole pores (cvp). Most importantly, the cell displays striking asymmetries. Cortical units align in rows parallel to the antero-posterior axis of the cell; on the ventral surface, the oral apparatus and cytoproct localize along an oral meridian, which defines the axis of right-left asymmetry, while the dorsal side is marked by the contractile vacuole pores. The global right-left asymmetry and antero-posterior polarity of the cell are expressed at the cortical unit level by the direction of the cytoskeletal appendages nucleated around each basal body such as the conspicuous ciliary rootlet ().
During division (), the organelles are duplicated and a wave of basal body duplication pervades the cell. Like centrioles in centrosomes, basal bodies duplicate by a conservative process with each neo-formed organelle arising close and at right angles to the mother. The process obeys a strict polarity: the new basal body inserts in the cortex just anterior to its “mother,” along the axis of the longitudinal row ( and B).Citation4 Cytoskeletal appendages will then be nucleated at precise sites of the “peribasal body material” within the limits of the cortical unit (). Although orchestrated at the whole cell level and triggered by the same mitotic signals, basal body duplication and the correlative duplication of cortical units are managed locally, at the cortical unit level ().
The pioneer experimental study started with doublet cells. “Doublets” had long been observed to appear occasionally in cultures of different ciliate species, and their stability was noted. Paramecium doublets comprise two complete sets of organelles and basal body fields, arranged in tandem (). This doublet organization is perpetuated through vegetative division and Sonneborn explored the genetic determination of this stability.
One advantage of Paramecium for genetic studies lies in the fact that each pair of conjugants simultaneously achieves the two reciprocal crosses, as conjugation involves only nuclear exchange: each conjugant retains its cellular integrity and a stationary, “female,” gametic nucleus which fuses with a migratory, “male,” gametic nucleus provided by the partner. Each pair of conjugants thus yields two F1 clones of identical heterozygous nuclear genotype but each retains its cytoplasmic characters. Occasional cytoplasmic bridges allow cytoplasmic exchanges, which can be monitored by cytoplasmically controlled characters such as the presence or absence of “κ particles,” a bacterial endosymbiont, or the alternative expression of mating types.Citation5 Doublets arise precisely when such bridges between conjugating cells fail to break, leading to complete fusion of the two conjugants.
Doublets could be crossed with normal partners carrying genetic nuclear and cytoplasmic markers. The doublet phenotype was maternally inherited and maintained in F2 and subsequent sexual generations in the maternal line of descent, regardless of the segregation of the genetic markers, and even after intermixing of endoplasm and cytoplasmic organelles, thus ruling out both nuclear and cytoplasmic determinants. Still, it could be argued that the singlet/doublet alternative might reflect alternative differentiated states of the macronucleus (a process known to control expression of Paramecium surface antigens and mating type) or that doublets might have a double gene complement. Both counter-arguments could be ruled out by sophisticated manipulations amounting to reciprocal transplantations of nuclei.Citation1
If the hereditary determinant for the doublet condition was neither in the nuclear genes nor in endoplasmic “information,” its basis ought to reside in the cortex itself, which would thus ensure its own perpetuation. This “cortical inheritance” suggested the more general concept of “cytotaxis” or “structural inheritance,” namely the directive role of preexisting structures and organization on assembly and organization of new structures.Citation6,Citation7
Structural Inheritance in Ciliates: Theme and Variations
The study of more discrete cortical mutants provided some insight into the mechanisms underlying cortical inheritance. Variant cells were spotted by their abnormal “twisty” swimming, a clonally inherited property which turned out to reflect the presence of a complete ciliary row with 180° reversed polarity as judged by the orientation of the ciliary rootlets along this row (). In order to understand the origin and stability of this discrete phenotypic change, similar changes were experimentally generated by grafting a small piece of cortex in reverse polarity. Cytological analysis of a number of such “grafted cells” showed: (1) soon after surgery, the presence, as expected, of a few intercalated short segments of ciliary rows with reversed polarity; (2) the progressive elongation of these intercalated segments in the course of the next two-three divisions; (3) the establishment of complete intercalated ciliary rows of reverse polarity in sub-clones of the parent cell derived from the posterior product of the first division following the graft. All these lines manifested the “twisty” swimming behavior, all the more pronounced, as the number of inverted rows was greater.Citation7 Cell lines with 2–3 to up to 12 inverted rows (among the ca. 70 normal longitudinal rows) were obtained. These cell lines retained their inverted rows for hundreds of cell divisions, with occasional loss of inverted rows. As in the case of doublets, genetic analysis ruled out any genetic change. The the autonomous polarity of duplication of basal bodies and cortical units. They carry and transmit information not only for assembly of organelles, but also for their polarity which thus appears not to be directly genetically determined. The morphogenetic autonomy of basal bodies and cortical units, the building blocks of the cortex, then also accounts for the cortical inheritance of the doublet condition.
An obvious question is: might these hereditary variations reflect some specificity of Paramecium, owing to its highly constrained mode of division, with strict conservation of all existing cortical structures and formation of new organelles, oral apparatus and contractile vacuole pores, close to preexisting cortical structures? Among the wide world of ciliates, species display different morphogenetic strategies and differ in regulative capacities. Thus “Paramecium is a ciliate counterpart to a mosaic embryo, whereas Tetrahymena is more like a regulative embryo.”Citation9
Nevertheless, 180° inverted rows were produced in Tetrahymena by the same type of micro-surgical method as in Paramecium and their hereditary maintenance demonstrated.Citation10 Both in Tetrahymena and in a related species, Glaucoma, further variant cortical configurations-mirror-image doublets and singlets displaying a reversal of cellular handedness relative to the antero-posterior cell axis-were also shown to be clonally inherited.Citation11,Citation12 Spirotrich ciliates like Oxytricha are still more highly regulative. They dedifferentiate and redifferentiate their ciliature at each division and can encyst, then loosing any ultrastructurally identifiable remnant of ciliature- associated structures.Citation13 However, upon excystment, they will redevelop their exact pre-cyst ciliature. So did doublets, whether homopolar or heteropolar, or cells possessing supernumerary dorsal structures: they all recapitulated their pre-cyst organization upon excystment.Citation14 Although in these case, genetic analysis was limited to homopolar doublets, the mode of origin and reversion of these variants indicated a nongenetic determinism.Citation14
Beyond evidence for a structural memory what do these experiments demonstrate? Firstly, no one-to-one correspondence links genotype and cell architecture since the genotype of a cilate can stably accommodate a range of variations departing from the wild type. Secondly, and this was the more provocative aspect, the guiding role of the existing organization on the assembly of new structures implies transmission of a new type of information, non-DNA-based. The term of “protein-based inheritance” was not then used, but it was pointed out that the presence of nucleic acids in basal bodies or any component of the cortex could not account for the observed phenomena, which do not bear on the properties of basal bodies or associated structures, but on variations in the spatial relationships among these elementary structures which themselves appear molecularly unaltered.Citation7 Later on, the persisting belief in of basal body-associated DNA was eventually ruled out.Citation15
Structural Inheritance and Basal-Body Biogenesis
The concept of structural inheritance derives of observations made on hereditary variations in the organization of the cortex of ciliates. Ciliate cortex is characterized by a defined, speciesspecific, spatial arrangement of basal bodies that, in turn, organize the cytoskeleton and control the whole cell architecture. During division, a precise spatio-temporal control of basal body duplication is required to restitute the parental organization in the two daughter cells.Citation3 Therefore, at least part of the determinism of cortical inheritance depends on the mechanism of basal body duplication and is likely to involve mechanisms of general biological significance. It happens that the centriolar structure, whether centriole or basal body, is the only cellular entity besides chromosomes that duplicates, and typically does so once per cell cycle. The stereotyped geometry of this duplication, with the new structure developing close to and at right angles to the mother suggests a templating mechanism ( and B). The underlying molecular processes are not yet fully dissected (), but in most cases, no such structure develops in the absence of a preexisting one. However, under particular biological conditions, a centriolar structure can form “de novo,” for example in the development of the flagellated gametes of certain plants, in the flagellate form of the amoebo-flagellate Naegleria or during differentiation of ciliated epithelia in metazoa.Citation16–Citation18 De novo formation of basal bodies or centrioles have also been experimentally induced in Chlamydomonas and HeLa cells by suppression of the resident centriole or in the unfertilized Drosophila egg which lacks centrioles.Citation19–Citation22 The presently accepted notion is that both the “templated” and the “de novo” pathways have been conserved and coexist, but that, in the presence of a pre-existing structure, the templated pathway prevails as “kinetically dominant” and represses the alternative mode.Citation19 Although convenient, the templated versus de novo terminology is misleading as it suggests the existence of a structural template provided by the mother which would be required in one case and dispensable in the other. The steps to assembly of a centriolar structure which begin to be unraveled () are likely to be similar in the two cases and the templating function of the mother organelle essentially lies in the control of the site and time of nucleation of the daughter organelle.Citation22–Citation25
It is remarkable that the templated mode of duplication of centriolar structures, which represents the paradigm of structural inheritance, appeared with the first eukaryote and has been conserved throughout evolution. There are several reasons for this conservation of the properties of organelles, which are the organizers of the cytoskeleton. First, the templated pathway achieves the conservation of number, a crucial parameter in cellular division; then it ensures a precise localization of the daughter organelle and thus contributes to the reproduction of cell organization; last but not least, it ensures transmission of polarities through cell divisions, a function which is conspicuous in ciliates or flagellates and is likely to accompany duplication of centrioles.Citation26 In fungi, all the functions of centrioles are fulfilled by a different organelle, the spindle pole body whose duplication, by direct budding at a preformed site of the mother organelle, also depends on structural inheritance and transmits polarities from mother cell to the bud.
Since an important function of centriolar structures is to nucleate a cilium or a flagellum, cilia and flagella might also be expected to express and transmit polarities since their circumferential polarities reproduce those of the basal body. In a remarkable instance, described in T. brucei, the transmission of cell shape and polarity is precisely mediated by a direct connection between the developing new flagellum and the old one.Citation27,Citation28 In metazoa, a role of primary cilia in the establishment of asymmetries and polarities during development has been established, as for determination of right-left asymmetry or for the functioning of the hedgehog or Wnt signaling cascade regulating growth and patterning.Citation29–Citation33
Finally, there may be more to basal body duplication than the organelle itself and its appendages, and structural inheritance may turn out to tell a Russian doll story. As noted above, the cysts of the spirotrich Oxytricha retain a blueprint of their cortical pattern. So do another spirotrich, Paraurostyla, and the cysts it forms during sexual processes, called zygocysts. An antibody raised against mammalian centrosomes was found to decorate, in the zygocyst, a transient system of tracks along which basal bodies redistribute upon excystment.Citation34 A recent study of Chlamydomonas reveals the existence of a filamentous centrin-containing scaffold that extends from the nucleus to the flagellar bases.Citation35 Such a centrin-based transcellular scaffold might contribute to embody the cellular geometry and might underlie the well-established role of these Ca2+-binding proteins in duplication of centrioles, basal-bodies and spindle pole bodies.Citation36 The idea also fits results obtained in Paramecium, which showed that basal-body-associated centrins control the geometry of basal body duplication, i.e., the site of assembly of the daughter basal body.Citation37 Centrin (or a centrin-containing scaffold associated with the basal body) has recently been shown to be required for duplication and positioning of the Golgi stack in Trypanosome.Citation38 Such scaffolds subtending the cell surface might have a general role in transmission of polarities, not only in cells, but also through development. A striking evidence for the existence of predetermined polarities was demonstrated in the development of the quail oviduct, where assembly of ciliary basal bodies represent the last step of differentiation: in a 180° inverted segment of oviduct primordium replaced in situ, basal bodies differentiate and grow cilia that beat with reversed polarity.Citation39 Inheritance of polarities—possibly mediated by fibrillar scaffolds—thus appears as a key parameter in cell memory with centriolar structures as major relays.
Structural Inheritance and the Limits of Direct Gene Control
The existence of structural inheritance challenges the idea that cellular organization is under direct gene control. This notion, which is a mere extension of the central dogma, implies that the properties of the proteins themselves are sufficient for their “self-assembly” into high order sub-cellular structures, such as cytoskeletal organelles and networks. In fact, the concept of self-assembly, where self-assembled proteins reach a stable equilibrium, as in the case of a virus capsid assembly, cannot account for the dynamic properties of cytoskeletal structures, which never reach a stable equilibrium. To accommodate the properties of versatile cytoskeletal networks in vivo, the concept of “self-organization” describes a stable albeit dynamic structure.Citation40–Citation42 However, if the specificities of the constitutive proteins and the dynamic properties of “self-organized” cytoskeletal polymers can generate both intrinsic polarity and pattern diversity, they still do not account for the three-dimensional-deployment of the networks within the cellular space. At the cellular level, the organization of the most general cytoskeletal constituent, microtubule networks, is determined by the existence of microtubule organizing centers (MTOCs), which provide focal sites of microtubule nucleation and determine microtubule polarity. The spatial deployment of the microtubule array can be explained by the “dynamic instability” of these polymers: switches between phases of growth and shrinkage allow random patterns to become fixed by stabilization at distal capture sites. This process of “dynamic instability” allows the network to display more than one organization but also an organization which is not directly defined by genetic information, but determined by the position of the MTOC and that of distal preexisting spatial cues, at kinetochores, membranes or cortex.Citation40 These degrees of freedom in pattern details mark the limits of gene control and underscore the determining role of preexisting spatial cues.
Even less dynamic, permanent, networks escape direct gene control of their pattern. An interesting example is again offered by Paramecium. The infraciliary lattice (ICL) is the innermost cytoskeletal network that subtends the cortex all over the cell: it forms a continuous net whose polygonal meshes, made of bundles of microfilaments, run around the bases of basal bodies (). The ICL is a contractile network, composed of numerous different centrins and of centrin-binding proteins.Citation43–Citation46 Upon Ca2+ entry, this massive array of high-affinity Ca2+-binding proteins causes cell contraction and correlative buffering of the deleterious action of the Ca2+ ions. Under laboratory conditions, the network is dispensable: inactivation of any of the centrin genes specific to the network leads to its disassembly, with no other effect on the cells than loss of contraction under a mild Ca2+-stress.Citation44 In stably ICL-less cells, dots of centrin-containing material (which appear ultrastructurally amorphous in contrast to ICl meshes) are retained within each cortical unit (). These dots always flank the same side of the basal body, and are “reproduced” with basal bodies, since they are consistently present next to basal bodies in the course of cell divisions. Reactivation of the silenced gene(s) leads to ICL reassembly. The time course of the process () shows that filament bundles are initiated only at the centrin-containing dots which we have called ICLOCs (for ICL-organizing centers), whose molecular components, besides centrins, are yet uncharacterized. The reassembly process presents two remarkable features: (1) at random places over the cell, short filaments emerge from an ICLOC; and (2) their elongation follows a random path until they merge into a continuum that eventually is stabilized into a pattern resembling the original one.Citation44 As for the organization of a microtubule array, the pattern of the ICL is not genetically programmed, but globally constrained by external preexisting spatial cues, and in particular by the position of the ICLOCs, which in turn reflects the basal body pattern... another Russian doll story.
The Tool Box of Structural Inheritance
Although we do not know how the ICL fibers are nucleated by the ICLOCs, the regeneration of the ICL in Paramecium reveals that self-sustained specialized organizing centers exist for cytoskeletal components other than the ubiquitous microtubules. But even to nucleate microtubules, cells have other resources than the trio of canonic MTOCs i.e., centrosome, spindle pole body and basal body. A simple device was first described in trypanosome where very short microtubules intercalate in the preexisting cortical array, as if nucleated by direct lateral interaction with existing adjacent microtubules, leading to a semi-conservative reproduction of the pattern.Citation47 A similar mechanism operates in plant cells where the extant cortical microtubules themselves bind γ-tubulin leading to a microtubuledependent microtubule nucleation process, which maintains the global organization and polarity of the cortical microtubule layer through longitudinal cell growth and division.Citation48 In Paramecium, devoid of centrosomes, aggregates of short microtubules and g-tubulin complexes reside permanently within the germline micronuclear compartment, where they partition at division to organize the mitotic spindle.Citation49
If centriolar structures and other self-maintained nucleation centers represent the basic tools of structural inheritance, perpetuation of cell organization also resorts to an array of devices which do not involve permanent organelles or molecular complexes but a diversity of more or less stable or transitory relays which nevertheless contribute to ordering of new by old cell structure/polarity across division. In Drosophila during ovogenesis, a special membranar structure, the fusome (a germline-specific organizer of mitotic spindles), present only over a small number of cell divisions, relays polarity from cystoblast to oocyte.Citation50 Similar situations can also be found in a “simple” unicell, S. cerevisiae. A well dissected polarity cue which marks the bud site and determines the axis of cell growth involves in particular Rax1p and Rax2p: these integral membrane proteins remain stably localized at the budding site through multiple cell cycles where they interact with the other proteins involved in the budding process.Citation51,Citation52 Large immobile protein complexes, called eisosomes, have recently been described; they comprise both cytoplasmic and membrane proteins and their stability is thought to be important for maintaining organization of the plasma membrane throughout cell divisions.Citation53 More generally, during cell division and development, if molecular traffic is controlled upstream by gene encoded zip codes in messenger RNAs or proteins, it is eventually dependent upon preexisting spatial cues and anchors that are most often localized at the cell surface that is at membranes which never form de novo. It is finally striking to note that polarities are embedded in centriolar structures, transmitted to cilia and their membrane which in turn play a role in spatial organization in the course of development.
Structural Inheritance as Protein Based-Inheritance
What is finally the fundamental meaning of the cortical inheritance demonstrated in ciliates and of the derived concept of structural inheritance? As first pointed out by Grimes and Aufderheide, it is basically of the same nature as prions which show that the same gene product can exist under two (or more) conformations whose accidental shift leads to new infectious and cytoplasmically transmissible phenotypes.Citation54 In structural inheritance, the observed variations do not affect protein conformation but rather the spatial arrangement of subcellular structures. However, in both types of phenomena, key steps involve nucleation processes and assembly of fibrous structures. At both molecular and subcellular levels, conformation is therefore not univocally determined by genetic information and the heritability of novel conformations of stochastic occurrence “widens the phenotypic space encoded by a given genome… and can modify the adaptive landscape and influence evolution.”Citation55 Considering that ciliary/flagellar basal bodies appeared with the first eukaryotes, it is hard to imagine that cortical variants with loss, addition or inversion of basal body territories may not have contributed at least to protist diversity.
From a historical point of view, it is interesting to note that when the initial work on Paramecium was published (1963–1965), the concept of structural inheritance had little impact and the cortical variants were often viewed as a zoological curiosity of limited significance. In fact, even though it was buttressed by a convincing genetic analysis, the demonstration that preformed cell structure played a key role in cell heredity encountered the same type of ideological barrier as the recognition of the “protein-only” status of prions. This experimental evidence for a protein-based cell memory was rarely integrated in biological research and this may in part explain why so few recognized cases of structural inheritance have been reported or explored: molecular or structural landmarks which provide evidence of even a short-term cell memory in the course of differentiation and development are not perceived as such but as a particular step along a linear sequence of reactions starting from the genes.
With the renewal of interest for epigenetic phenomena, and the unlimited possibilities offered by all the new “omics” and tools for functional analysis, many more landmarks, anchors, and structural relays involved in division and development will be characterized, and reveal the molecular circuitry underlying cell memory which essentially stores polarity landmarks and cues for spatial organization during division and development.
Figures and Tables
Figure 1 Cortical organization of Paramecium. The figure shows the ventral (left) and dorsal (right) sides of a cell immunolabeled by an anti-tubulin antibody which reveals basal bodies as discrete dots (bb) and an antibody directed against the ciliary rootlets (cr) which form a thin bundle emanating from each basal body, as shown in the enlargement. A-P marks the antero-posterior cell axis. The ventral side is marked by a line of contrast in the global arrangement of basal body rows, the oral meridian, which defines the right (R) and the left (L) of the cell. The oral apparatus (oa)-a ciliated funnel at the bottom of which phagocytosis takes place-and the cytoproct (cy) open on the ventral side, while the pores of the contractile vacuole systems (cvp) localize on the dorsal surface. Bar: 10 mm. Images appear courtesy of F. Ruiz.
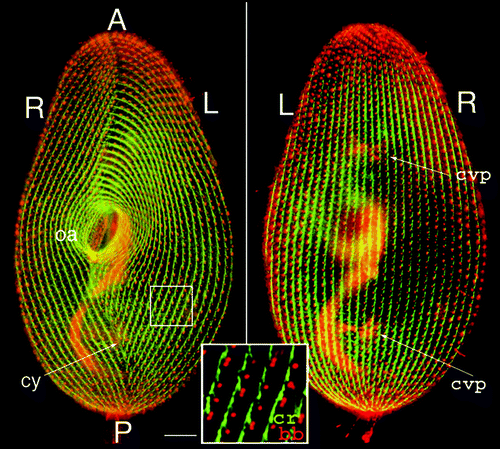
Figure 2 Morphogenetic processes during division. The figure shows interphase and dividing living cells expressing GFP-PtCen2a, a centrin specific to basal bodies.Citation29 Smaller dots correspond to a single basal body per cortical unit, larger ones to two basal bodies per unit. In the dividing cell, where basal body duplication proceeds, the division furrow (df) delimits the two presumptive daughter cells. The old oral apparatus is conserved in the anterior daughter cell, while a new one has developed in the posterior one. The two insets pinpoint three units with 2 bbs in the interphase cell and their progeny in the dividing cell. Images are shown with reverse contrast. Bar: 10 mm. Images appear courtesy of F. Ruiz.
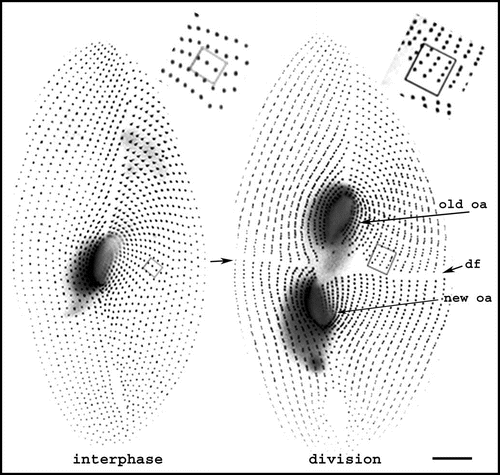
Figure 3 Basal body duplication. (A) Polarity of basal body duplication in Paramecium. Each new basal body (new bb), develops at right angles and anterior to its mother (old bb), then tilts up to become inserted in the cortex, along the same basal body row as the mother organelle.Citation4 Fibrous links connect mother and daughter basal bodies. (B) Basal body duplication in situ. This electron microscopic view of basal body duplication shows two old bbs in cross-section and two new bbs (thick arrows), still in orthogonal position, anterior to the old bbs and aligned along the row. The A-P and R-L polarities of the rows are indicated: these polarities are indicated by the position and orientation of two basal body appendages, the ciliary rootlets (thin arrows) and a microtubule ribbon (arrowheads). Bar: 100 nm. Image: courtesy of N. Garreau de Loubresse. (C) The assembly line. The scheme takes into account recent data on the molecular dissection of basal body/centriole assembly in C. elegans, Drosophila and human cells and depicts the likely general stepwise scaffolding process leading to assembly of a centriole/basal body. The earliest detectable seed is a “central tube”, forming the axis of the cartwheel corresponding to the onset of the ninefold symmetry. Then at the apex of the 9 radii, the sequential assembly and elongation of microtubule will result in the final cylinder of microtubule triplets. The same sequence is likely to take place in both templated and de novo pathway.Citation20–Citation23,Citation25,Citation26 A central tube appears as the first identified seed of the organelle; the 9-branched star symbolizes the required prepattern for the characteristic ninefold symmetry of the microtubule cylinder which elongates along with the sequential addition of tubules A–C.
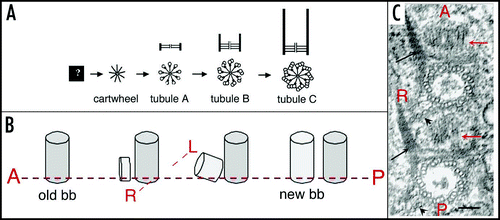
Figure 4 Duplication of the cortical pattern. (A) The elementary cortical unit. The scheme shows a basal body and its main cytoskeletal appendages whose extension defines the cortical unit. The striated ciliary rootlet (cr) runs anterior and to the right of the basal body and of the cell; the transverse microtubule ribbon (tmr) and the post-ciliary microtubule ribbon (p-cmr) run to the left and the posterior sides of the basal body respectively. (B) Duplication of the cortical units. Two adjacent units along two parallel rows are represented. Solid lines correspond to old bb and appendages, dotted lines to new organelles. Elongation of the longitudinal rows proceeds through elongation of the preexisting units and intercalation of new bbs.
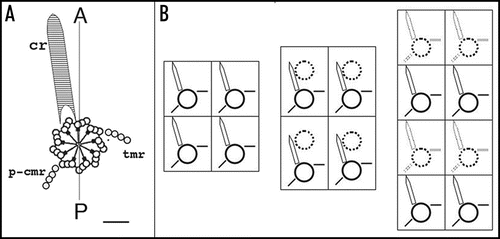
Figure 5 Organization of Paramecium doublet cells. The upper drawings outline a singlet and a homopolar doublet. In the singlet, the oral apparatus (oa) faces the observer and contractile vacuole pores (cvp) are on the hidden face. In the doublet, the second oral apparatus (oa2) is on the hidden side, while the corresponding contractile vacuole pores (cvp2) face the observer. The lower drawings represent the corresponding equatorial cross-sections, allowing a better visualization of the tandem organization of the doublet. R, L, V, D mark right, left, ventral, and dorsal sides of the singlet, respectively; R1/R2, V1/V2, etc. localize the different fields of the fused cells in the doublet.
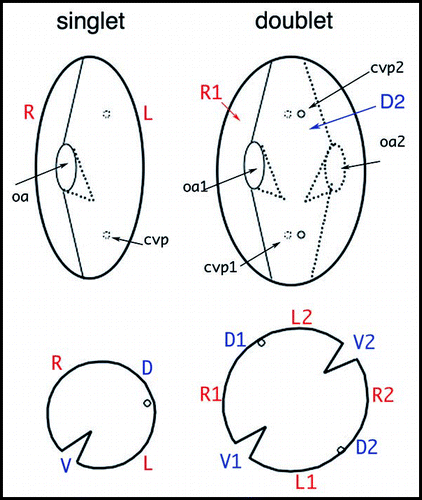
Figure 6 Visualization of an inverted ciliary row. As in , double staining of basal bodies and ciliary rootlets indicates the polarity of basal bodies and cortical units. On the left panel, the arrow points to a discontinuity in the cortical pattern. On the right panel, the enlargement around this discontinuity shows the direction of the ciliary rootlets, which run downward and to the right of the observer in the case of the inverted row, while they run upward and to the left of the observer for normally oriented rows. Bar: 10 mm. Image appears courtesy of F. Ruiz.
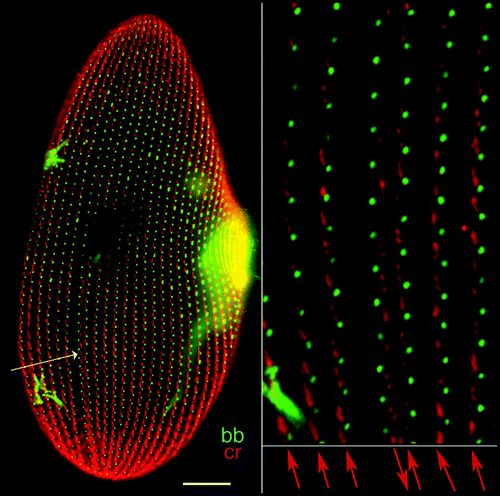
Figure 7 Organization of the infraciliary lattice. This cytoskeletal network is composed of centrins and centrin-binding proteins associated in bundles of microfilaments. The lattice is labeled by anti-centrin antibodies (in green). (A) In a wild type cell, the ICL network subtends the whole cell and each mesh encircles a basal body (labeled in red by an anti-tubulin antibody). (B) In cells depleted of one of the constitutive centrins by RNAi treatment, the network is disassembled, but cortical units retain dots of anti-centrin reactive material flanking the basal bodies. Bar: 10 mm.
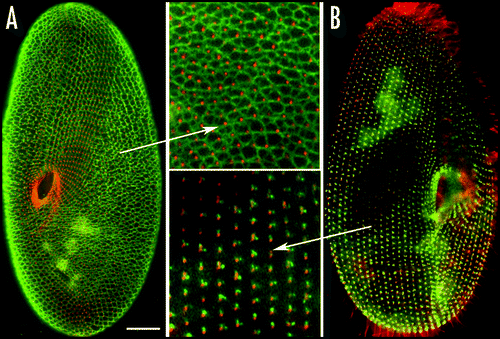
Figure 8 Mode of reassembly of the infraciliary lattice. Upon release of the silencing conditions, filaments arise from the residual dots flanking the basal body. The four images in black end white show, from left to right, the progress of reassembly over 1–3 divisions; and the network does not recover its normal pattern (rightmost image) until 10–15 divisions. Bar: 2 mm.

Note
This manuscript has been previously published: Centriole Wilson P. Chernoff Y. Inheritance. Protein-Based Inheritance 2007; Austin and New York Landes Bioscience and Kluwer Academic Press 106 - 118
References
- Sonneborn TM. Allen JM. Does preformed cell structure play an essential role in cell heredity?. The Nature of Biological Diversity 1963; New York McGraw-Hill 165 - 222
- Griffith JS. Self-replication and scrapie. Nature 1967; 215:1043 - 1044
- Iftode F, Cohen J, Ruiz F, Torrès-Rueda A, Chen-Shan L, Adoutte A, Beisson J. Development of surface pattern during division. I. mapping of duplication and reorganization of cortical cytoskeletal structures in the wild type. Development 1989; 105:191 - 211
- Dippell RV. The development of basal bodies in Paramecium. Proc Natl Acad Sci USA 1968; 61:461 - 468
- Sonneborn TM. Partners of the genes. Scient Amer 1950; 30 - 39
- Sonneborn TM. The differentiation of cells. Proc Natl Acad Sci US 1964; 51:915 - 929
- Beisson J, Sonneborn TM. Cytoplasmic inheritance of the organization of the cell cortex in Paramecium aurelia. Proc Natl Acad Sci US 1965; 53:275 - 282
- Tamm SL, Sonneborn TM, Dippell RV. The role of cortical orientation in the control of the direction of ciliary beat in Paramecium. J Cell Biol 1975; 64:98 - 112
- Frankel J. Structural inheritance. Pattern Formation: Ciliate Studies And Models 1989; Oxford Oxford University Press 69 - 93
- Ng SF, Frankel J. 180° rotation of ciliary rows and its morphogenetic implications in Tetrahymena pyriformis. Proc Natl Acad Sci USA 1977; 74:1115 - 1119
- Nelsen EM, Frankel J, Jenkins LM. Nongenic inheritance of cellular handedness. Development 1989; 105:447 - 456
- Suhama M. Reproducing singlets with an inverted oral apparatus in Glaucoma scintillans (Ciliophora, Hymenostomatida). J Protozool 1985; 32:454 - 459
- Grimes GW. Morphological discontinuity of kinetosomes during the life cycle of Oxytricha fallax. J Cell Biol 1973; 57:229 - 232
- Hammersmith RL, Grimes GW. Effects of cystment on cells of Oxytricha fallax possessing supernumerary dorsal bristle rows. J Embryol Exp Morphol 1981; 63:17 - 27
- Johnson KA, Rosenbaum JL. Basal bodies and DNA. Trends Cell Biol 1991; 6:145 - 149
- Wolniak SM, Klink VP, Hart PE, Tsai CW. Control of development and motility in the spermatozoids of lower plants. Gravit Space Biol Bull 2000; 13:85 - 93
- Fulton C, Dingle AD. Basal bodies but not centrioles, in Naegleria. J Cell Biol 1971; 51:826 - 836
- Anderson RG, Brenner RM. The formation of basal bodies (centrioles) in the Rhesus monkey oviduct. J Cell Biol 1971; 50:10 - 34
- Marshall WF, Vucica Y, Rosenbaum JL. Kinetics and regulation of de novo centriole assembly: Implications for the mechanism of centriole duplication. Curr Biol 2001; 11:308 - 317
- La Terra S, English CN, Hergert P, McEwen BF, Sluder G, Khodjakov A. The de novo centriole assembly pathway in HeLa cells: Cell cycle progression and centriole assembly/maturation. J Cell Biol 2005; 168:713 - 722
- Peel N, Stevens NR, Basto R, Raff JW. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol 2007; 17:834 - 843
- Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science 2007; 316:1046 - 1050
- Pelletier L. Centrioles: Duplicating precariously. Curr Biol 2007; 17:770 - 773
- Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol 2007; 176:173 - 182
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell 2007; 13:190 - 202
- Beisson J, Jerka-Dziadosz M. Polarities of the centriolar structure: Morphogenetic consequences. Biol Cell 1999; 91:367 - 378
- Moreira-Leite FF, Sherwin T, Kohl L, Gull K. A trypanosome structure involved in transmitting cytoplasmic information during cell division. Science 2001; 294:610 - 612
- Briggs LJ, McKean PG, Baines A, Moreira-Leite F, Davidge J, Vaughan S, Gull K. The flagella connector of Trypanosoma brucei: An unusual mobile transmembrane junction. J Cell Sci 2004; 117:1641 - 1651
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998; 95:829 - 837
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA 2005; 102:11325 - 11330
- Singla V, Reiter JF. The primary cilium as the cell's antenna: Signaling at a sensory organelle. Science 2006; 313:629 - 633
- Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic 2007; 8:97 - 109
- Beales PL. Hedgehogs on the road to polarity. Nat Genet 2006; 38:277 - 279
- Fleury A, Le Guyader H, Iftode F, Laurent M, Bornens M. A scaffold for basal body patterning revealed by a monoclonal antibody in the hypotrich ciliate Paraurostyla weissei. Dev Biol 1993; 157:285 - 302
- Geimer S, Melkonian M. Centrin scaffold in Chlamydomonas reinhardtii revealed by immunoelectron microscopy. Eukaryot Cell 2005; 4:1253 - 1263
- Rice LM, Agard DA. Centriole duplication: Centrin in on answers?. Curr Biol 2002; 12:618 - 619
- Ruiz F, Garreau de Loubresse N, Klotz C, Beisson J, Koll F. Centrin deficiency in Paramecium affects the geometry of basal-body duplication. Curr Biol 2005; 15:2097 - 2106
- He CY, Pypaert M, Warren G. Golgi duplication in Trypanosoma brucei requires Centrin2. Science 2005; 310:1196 - 1198
- Boisvieux-Ulrich E, Sandoz D. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol Cell 1991; 72:3 - 14
- Kirschner M, Gerhart J, Mitchison T. Molecular “vitalism”. Cell 2000; 100:79 - 88
- Kushner DJ. Self-assembly of biological structures. Bacteriol Rev 1969; 33:302 - 345
- Kirschner M, Mitchison T. Beyond self-assembly: From microtubules to morphogenesis. Cell 1986; 45:329 - 342
- Klotz C, Garreau de Loubresse N, Ruiz F, Beisson J. Genetic evidence for a role of centrin-associated proteins in the organization and dynamics of the infraciliary lattice in Paramecium. Cell Motil Cytoskeleton 1997; 38:172 - 186
- Beisson J, Clérot JC, Fleury-Aubusson A, Garreau de Loubresse N, Ruiz F, Klotz C. Basal body-associated nucleation center for the centrin-based cortical cytoskeletal network in Paramecium. Protist 2001; 152:339 - 354
- Gogendeau D, Beisson J, de Loubresse NG, Le Caer JP, Ruiz F, Cohen J, Sperling L, Koll F, Klotz C. An Sfi1p-like centrin-binding protein mediates centrin-based Ca2+-dependent contractility in Paramecium tetraurelia. Eukaryot Cell 2007; 6:1992 - 2000
- Gogendeau D, Klotz C, Arnaiz O, Malinowska A, Dadlez M, Garreau de Loubresse N, Ruiz F, Koll F, Beisson J. Functional diversification of centrinsand cell morphological complexity. J Cell Sci 2008; 121:65 - 74
- Sherwin T, Gull K. Visualization of detyrosination along single microtubules reveals novel mechanisms of assembly during cytoskeletal duplication in trypanosomes. Cell 1989; 57:211 - 221
- Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, Horio T, Hasebe M. Microtubule-dependent microtubule nucleation based on recruitment of g-tubulin in higher plants. Nat Cell Biol 2005; 7:961 - 968
- Klotz C, Ruiz F, Garreau de Loubresse N, Wright M, Dupuis-Williams P, Beisson J. Gamma-tubulin and MTOCs in Paramecium. Protist 2003; 154:193 - 209
- Huynh JR, St Johnston D. The origin of asymmetry: Early polarisation of the Drosophila germline cyst and oocyte. Curr Biol 2004; 14:438 - 449
- Kang PJ, Angerman E, Nakashima K, Pringle JR, Park HO. Interactions among Rax1p, Rax2p, Bud8p, and Bud9p in marking cortical sites for bipolar bud-site selection in yeast. Mol Biol Cell 2004; 15:5145 - 5157
- Chen T, Hiroko T, Chaudhuri A, Inose F, Lord M, Tanaka S, Chant J, Fujita A. Multigenerational cortical inheritance of the Rax2 protein in orienting polarity and division in yeast. Science 2000; 290:1975 - 1978
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature 2006; 439:998 - 1003
- Grimes G, Aufderheide KJ. Cellular aspects of pattern formation: The problem of assembly 1991; Karger (Basel)
- Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet 2005; 6:435 - 450