Abstract
Cellular prion protein (PrPC) appears to be involved in numerous physiological processes. We have recently shown a novel modulation of NMDA receptors by PrPC that results in neuroprotection via silencing of NMDA receptors containing NR2D subunits, whereas no effects on AMPA receptor function could be observed (Khosravani et al. 2008, J Cell Biol. 181, 551). Here we show that PrP-null mice show a normal response to long-term depression stimuli requiring AMPA receptor activity, thus further supporting our previous findings of a selective action on NMDA receptors among ionotropic glutamate receptors.
The role of prion proteins in the pathophysiology of transmissible spongiform encephalopathies is well documented.Citation1 Although there is a growing body of literature associating normal cellular prion protein (PrPC) with functions such as regulation of cell proliferation and survival, cell signalling and immune function,Citation1 the spectrum of physiological roles attributable to PrPC remains to be determined. This may in part be due to the fact that mice lacking PrPC display a relatively mild phenotype, unless subjected to insults such as ischemia or seizures, where increased mortality of the PrP-null mice has been reported.Citation2–Citation5 Interestingly, the increased neuronal damage in PrP-null mice following excitotoxicity is alleviated upon treatment with the N-Methyl-D-Aspartate (NMDA) receptor (NMDAR) inhibitor MK-801,Citation6 suggesting a neuroprotective role of PrPC via an action on NMDARs, but the mechanism was unclear.
We recently described a novel action of PrPC on NMDAR function.Citation7 By examining the neurophysiological properties of hippocampal neurons isolated from PrP-null mice, we were able to show that PrP-null mouse neurons exhibit enhanced and drastically prolonged NMDA evoked currents due to a functional upregulation of NMDARs containing NR2D subunits. Biochemical analyses suggested that NR2D subunits, but not NR2B subunits, co-immunoprecipitated with PrPC, indicating that PrPC and NMDARs form physical signaling complexes in neurons. The increased NMDAR function could be phenocopied by RNA interference and were rescued upon overexpression of exogenous PrPC. The enhanced NMDAR activity resulted in increased neuronal excitability, as well as enhanced glutamatergic-based excitotoxicity in both in vitro and in vivo experiments were neurons were transiently exposed to the selective agonist NMDA. Hence, native PrPC appears to mediate an important neuroprotective role by virtue of its ability to silence NR2D containing NMDARs. In contrast, minor effects on amplitude and rise and decay-time kinetics were observed for both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and GABAA (miniature and evoked) currents in synaptically mature hippocampal cultures.
AMPA and NMDA receptors have been linked to synaptic plasticity, in particular long term potentiation (LTP) and long term depression (LTD). LTP is believed to mostly reflect a strengthening of the postsynaptic response, caused by a brief period of hyperexcitability that releases significant amounts of glutamate such as during a brief tetanic stimulation. This is thought to result in the opening of AMPA receptors, which depolarize the postsynaptic membrane. This in turn increases the activity of postsynaptic NMDARs, because magnesium ions that normally inhibit NMDAR activity, are dislodged by the postsynaptic depolarization, thus allowing NMDARs to become active. This functional activation of NMDARs results in the influx of calcium ions, which in turn initiate a signaling cascade that results in the membrane insertion of additional AMPA receptors, thus strengthening the synapse. This process is thought to involve NMDA receptor isoforms that predominantly contain the NR2A subunits.Citation8
A synaptic depotentiation process also can take place that results in the opposite effects of LTP; this process is known as long-term depression or LTD, which also has an NMDAR-dependent component. In contrast to brief tetanic stimulation, as is used in the induction of LTP, establishing LTD requires low frequency stimulation (e.g., 1 Hz for 15 min). Successful and repeatable induction of LTD depends on the parameters used for the conditioning stimulus and more importantly on the age of the animal. In juvenile animals (P12–P21) a low frequency protocol is effective and the mechanism of LTD is believed to depend on the activity of NMDA receptors containing NR2B subunits.Citation8 Although a clear distinction of the roles between NR2A and NR2B containing NMDARs, in LTP and LTD respectively, has remained controversial,Citation9 it is clear that both NR2A and NR2B are key mediators of alterations in synaptic plasticity. In older animals, the conditioning protocol is reported to require modification to include paired-pulses. This is thought to be due to the involvement of predominantly AMPA (and perhaps kainate) receptors in addition to mGluRs responsible for the synaptic depotentiation (reviewed in ref. Citation10).
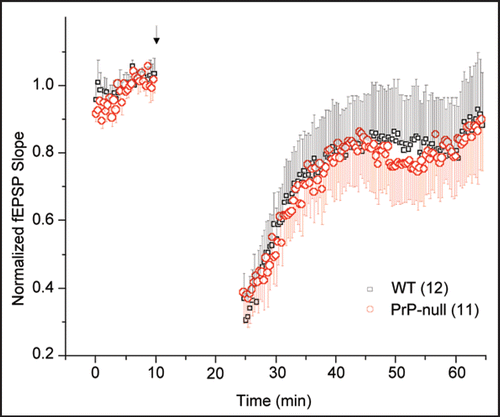
As mentioned earlier, our data obtained from hippocampal cultures indicated only a minor effect of PrPC knockout on AMPA receptor function. Hence, we hypothesized that AMPA receptor-mediated LTD should be similar in both wild type and PrP-null mice. We therefore examined the effect of PrP on LTD in hippocampal slices obtained from P30–P45 wild type mice and Zurich 1 PrPC knockout mice. Extracellular potentials were recorded using a patch pipette filled with 150 mM NaCl. First, 10 minutes of baseline evoked (every 30 sec) potentials were recorded to ensure stability of the preparation. LTD was then evoked by application of conditioning paired pulses (Δt = 60 ms) delivered at 1 Hz for 15 min. Thereafter, the field response was sampled every 30 sec for 40 min. As shown in , this protocol evoked reliable LTD in wild type mice that partially recovered over the time course of about 20 minutes (). In the PrPC-null slices, LTD was indistinguishable from that observed in the wild type slices ().
The age of the animals, combined with the paired pulse protocol used in our experiments was designed to isolate AMPA receptor mediated LTD.Citation10 The notion that LTD was unaltered in PrP-null mice is consistent with the observation that AMPA currents were not altered in these mice, and that AMPA receptor-mediated spontaneous synaptic events showed only minute changes compared with wild type animals. These data are also consistent with the notion that PrP-null mice show only mild phenotypes in spatial learning, with no apparent overall short-term memory deficits. Collectively, these data further support a selective action of PrP on NMDA receptors, rather than overall glutamatergic synaptic transmission.
Materials and Methods
Detailed methods for brain slice recordings from adult mice have been provided by us previously.Citation7 Briefly, adult male and female WT mice between P30–P45 were used for all slice experiments. Mice were anaesthetized with halothane and quickly decapitated. The brain was dissected and maintained in ice-cold artificial cerebrospinal fluid (aCSF) for approximately 1 min (bubbled with 95% O2 and 5% CO2). Horizontal hippocampal slices (∼250 µm) were obtained at an angle of approximately 12° in the frontal-occipital direction (Vibratome 1000, Vibratome Inc.) while submerged in ice-cool oxygenated aCSF. The slices were immediately transferred to a holding chamber where cool oxygenated aCSF was gradually warmed to 32°C at the end of the sectioning and maintained at that temperature for subsequent electrophysiological recordings. All slices were allowed a minimum of 1 hour of recovery prior to experimentation.
Slice recordings were performed in standard extracellular solution, ACSF containing (in mM): NaCl, 125; KCl, 5; NaH2PO4, 1.25; MgSO4, 2; CaCl2, 1.5; NaHCO3, 25; D-glucose, 10, pH 7.4 when bubbled with 95% O2 and 5% CO2. The osmolarity was 310 ± 5 mOsm. Field recordings were performed in the CA1 layer of the hippocampus. Evoked (orthodromic) field stimuli were derived using a bipolar simulating electrode placed in the Schaffer collaterals. The stimulus was generated by a constant voltage stimulator (DigiTimer, DS2A-MKII) with pulse delivery under computer control. The minimum stimulus intensity was obtained by noting the stimulus intensity required to evoke the first instance of a population spike using the first pulse. Maximum stimulus threshold was determined at the stimulus intensity at which the amplitude of the population spike reached a plateau. A total of 11 slices from three PrPC knockout animals, and 12 slices from four wild type animals were examined.
Figures and Tables
Figure 1 LTD in the CA1 region of hippocampal slices from adult (P30–P45) WT and PrP-null mice. The conditioning pulse (arrow head) was delivered as paired-pulses (Δt = 60 ms) at 1 Hz for 15 min at the Schaffer collaterals. Analysis of field excitatory postsynaptic potential (fEPSP) slope revealed no statistically significant differences (Student's t-test, p > 0.05) in the extent of induced LTD or the time course of its recovery to baseline. Numbers in parentheses indicate number of slices.
Acknowledgements
This work was supported by a grants from the Alberta Prion Research Institute and PrioNet Canada to G.W.Z. G.W.Z. is a Scientist of the Alberta Heritage Foundation for Medical Research (AHFMR) and a Canada Research Chair. H.K. is supported by studentships from the AHFMR and the Canadian Institutes of Health Research. We thank Dr. Frank Jirik for providing the PrP-null mice.
Commentary to:
References
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev 2008; 88:673 - 728
- Liang J, Bai F, Luo G, Wang J, Liu J, Ge F, Pan Y, Yao L, Du R, Li X, Fan R, Zhang H, Guo X, Wu K, Fan D. Hypoxia induced overexpression of PrP(C) in gastric cancer cell line. Cancer Biol Ther 2007; 6:769 - 774
- Walz R, Amaral OB, Rockenbach IC, Roesler R, Izquierdo I, Cavalheiro EA, Martins VR, Brentani RR. Increased sensitivity to seizures in mice lacking cellular prion protein. Epilepsia 1999; 40:1679 - 1682
- Turu M, Slevin M, Ethirajan P, Luque A, Elasbali A, Font A, Gaffney J, Cairols M, Kumar P, Kumar S, Krupinski J. The normal cellular prion protein and its possible role in angiogenesi. Front Biosci 2008; 13:6491 - 6500
- Weise J, Crome O, Sandau R, Schulz-Schaeffer W, Bahr M, Zerr I. Upregulation of cellular prion protein (PrPc) after focal cerebral ischemia and influence of lesion severity. Neurosci Lett 2004; 372:146 - 150
- Rangel A, Burgaya F, Gavin R, Soriano E, Aguzzi A, Del Rio JA. Enhanced susceptibility of Prnp-deficient mice to kainate-induced seizures, neuronal apoptosis and death: Role of AMPA/kainate receptors. J Neurosci Res 2007; 85:2741 - 2755
- Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, Chen L, Villemaire M, Ali Z, Jirik FR, Zamponi GW. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Cell Biol 2008; 181:551 - 565
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci 2004; 24:8885 - 8895
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology 2007; 52:71 - 76
- Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Prog Neurobiol 2001; 65:339 - 365