Abstract
In the past decade, the interaction between prions and nucleic acids has garnered significant attention from the scientific community. For many years, the participation of RNA and/or DNA in prion pathology has been largely ruled out by the "protein-only" hypothesis, but this is now being reconsidered. Experimental data now indicate that nucleic acids (particularly RNA), besides being carriers of genetic information, function as important key components during development, physiological responsiveness, and cellular signaling. This revelation has brought a new perspective to prion pathology. Here we discuss the role of RNA molecules in prion protein aggregation and the resulting cellular toxicity. We combine our most recent findings with existing literature to shed new light on this exciting field of research.
The “protein-only” hypothesis postulates that PrPSc ‘multiplies’ by catalyzing the conversion of PrPC into a molecule resembling itself, thereby leading to its own propagation.Citation1,Citation2 This theory has excluded the participation of nucleic acids in prion propagation. However, the suggestion that an additional unknown factor could influence the PrPC to PrPSc conversion has brought back the possibility of nucleic acid involvement in prion diseases, where evidence for this has been accumulating over the past decade.Citation3–Citation8 Instead of encoding genetic information, nucleic acid molecules would act by lowering the free energy barrier between PrPC and PrPSc and, consequently, triggering conversion.Citation9,Citation10
In the last decade, our group has studied the interaction between prion proteins and nucleic acids. We have demonstrated that PrP interacts with nucleic acids in vitro, binds small sequences of double stranded DNA, acquires β-sheet secondary structure according to spectroscopic measurements, and presents some PrPSc-like characteristics.Citation4,Citation11 Studies on the interaction of prion protein with RNA molecules have demonstrated that PrP can acquire protease resistance upon RNA binding, and that scrapie and cellular PrP isoforms bind with different affinities to some highly structured RNA sequences.Citation12–Citation15
In our latest report, we presented experimental data on the interaction between murine recombinant prion protein (rPrP23-231) and RNA molecules.Citation16 We used total prokaryotic and eukaryotic RNA extracts from cultured cells along with small synthetic oligonucleotides and looked for changes in the secondary and tertiary structures of PrP. We also used two mutants lacking the N-terminal domain to demonstrate the importance for RNA binding, and evaluated the toxicity of the PrP:RNA complex in cultured mouse neuroblastoma cells (N2a).Citation17
Our findings show that the heterogeneous mixture of RNA extracted from neuroblastoma cells was the only sample capable of triggering toxicity and massive aggregation. rPrP23-231 loses most of its secondary structure and immediately aggregates upon interaction with RNA. Interaction with small RNA sequences also leads to changes in the size of the complex and some aggregation, but the oligomeric species did not lead to toxicity. NMR investigations of this complex revealed that full length PrP partially recovers its native fold 72 h after RNA addition, and that the changes in the HSQC spectrum suggest that RNA binding causes some subtle changes in PrP structure. RNA does not induce aggregation of the PrP N-terminal deletion mutants, indicating that the N-terminal region is important for this process. We also observed that the rPrP23-231:N2aRNA complex protects both protein and RNA from degradation with proteinase K and Rnase A, respectively.
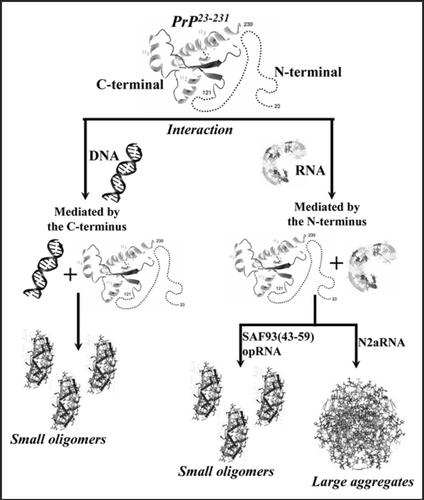
Moreover, we have seen that the PrP interaction with DNA, though identical in some respects, displays substantial differences from the interaction with RNA, and we try to correlate these differences with a possible physiological role for these binding events. We summarize the findings obtained when the prion protein binds DNA or RNA in . The heterogeneous mixture of RNA extracted from neuroblastoma cells was the only sample that triggered toxicity and massive aggregation. The small RNAs were able to bind PrP but formed only small, non-toxic oligomeric species.
The component of the N2a RNA extract that facilitates aggregation and induces toxicity remains unidentified. In additional, several other questions remain unanswered. How do PrP:RNA aggregates exercise their toxicity? Is there a cellular receptor for prion aggregates? Is the observed cellular death caused by apoptosis or necrosis? Is it possible that a non-self RNA could produce toxic aggregates in other cell types?
All of these questions are becoming more relevant with the emerging evidence that RNAs can participate as regulatory molecules.Citation18 The physicochemical properties of RNA allow it to participate in a diverse range of structural and catalytic roles through various mechanisms, including cell-to-cell communication.Citation19
Studies in plants first have shown that the phenomenon of co-suppression in response to transgene expression is mediated by RNA signaling and that this effect, which involves the RNA interference (RNAi) pathway, can be transmitted throughout the entire plant.Citation8,Citation20–Citation25 There is also evidence for systemic RNA signaling in animals. The discovery of RNAi in Caenorhabditis elegans showed that RNA signals can be transmitted both from the environment (via ingestion) and systemically throughout the organism.Citation26
RNA molecules can act as molecular adaptors to connect incoming analog signals to sequence-specific outcomes.Citation18 This is exemplified by ‘riboswitches,’ which are mRNA elements that alter their structure in response to ligands, enabling them to sense and react to environmental parameters.Citation27,Citation28 This capacity enables the creation of new classes of RNA signaling molecules that vary in their target specificity but utilize a common infrastructure and output, thereby providing latitude to explore new connections in RNA-based regulatory networks.Citation29,Citation30 These findings place the PrP:RNA interaction in a new context since RNA is no longer confined within the cell.
Since both aggregation and nucleic acid binding occur with decreased hydration, a bypass of the unfolded state can arise in the conversion of PrPc into a PrPSc-like structure.Citation11,Citation31 The interconversion might relate to an additional function of the PrP, such as a nucleic acid chaperone.Citation8 One of the main characteristics of a nucleic acid chaperone is the occurrence of mutual coupled rearrangements.Citation32 Nucleic-acid binding of the prion protein appears with the ordering and structural modification of the N-domain.Citation33 Thus, PrP might exert its function by participating as a NA chaperone in the gene regulation array at the post-transcriptional level, especially because of its capacity to bind both small RNAs and DNAs. In fact, PrP has been shown to interfere with the synthesis of HIV-1 proteins, implying an interaction with retroviral RNA.Citation34 The possibility of a functional role for PrP in nucleic acid processing has recently been corroborated by the demonstration that PrP participates in the control of activated endogenous retroviruses.Citation35 In the years ahead, we expect substantial progress in the breathtaking field of prion-RNA interactions.
Figures and Tables
Figure 1 Diagram summarizing the findings obtained when rPrP binds either DNA or RNA. While rPrP binds DNA mainly through the C-terminal domain (left), it binds RNA through its N-terminus (right). Small RNAs (SAF9343–59 and opRNA) bind to PrP but only form small, non-toxic oligomeric species in contrast to N2aRNA binding, which forms large oligomers.
Acknowledgements
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Millenium Institute for Structural Biology in Biomedicine and Biotechnology (CNPq Millenium Program), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
References
- Griffith JS. Self-replication and scrapie. Nature 1967; 215:1043 - 1044
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136 - 144
- Telling GC, Scott M, Mastriann J, Gabizon R, Torchia M, Cohen FE, DeArmond SJ, Prusiner SB. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 1995; 83:79 - 90
- Cordeiro Y, Machado F, Juliano L, Juliano MA, Brentani RR, Foguel D, Silva JL. DNA converts cellular prion protein into the beta-sheet conformation and inhibits prion peptide aggregation. J Biol Chem 2001; 276:49400 - 49409
- Nandi PK, Nicole JC. Nucleic acid and prion protein interaction produces spherical amyloids which can function in vivo as coats of spongiform encephalopathy agent. J Mol Biol 2004; 344:827 - 837
- Nandi PK, Leclerc E, Nicole JC, Takahashi M. DNA-induced partial unfolding of prion protein leads to its polymerisation to amyloid. J Mol Biol 2002; 322:153 - 161
- Caughey B, Kocisko DA. Prion diseases: a nucleic-acid accomplice?. Nature 2003; 425:673 - 674
- Silva JL, Lima LM, Foguel D, Cordeiro Y. Intriguing nucleic-acid-binding features of mammalian prion protein. Trends in Bioch Sci 2008; 33:132 - 140
- Baskakov IV, Legname G, Prusiner SB, Cohen FE. Folding of prion protein to its native alpha-helical conformation is under kinetic control. J Biol Chem 2001; 276:19687 - 19690
- Cordeiro Y, Silva JL. The hypothesis of the catalytic action of nucleic acid on the conversion of prion protein. Protein Pept Lett 2005; 12:251 - 255
- Lima LM, Cordeiro Y, Tinoco LW, Marques AF, Oliveira CL, Sampath S, Kodali R, Choi G, Foguel D, Torriani I, Caughey B, Silva JL. Structural insights into the interaction between prion protein and nucleic acid. Biochemistry 2006; 45:9180 - 9187
- Deleault NR, Lucassen RW, Supatapone S. RNA molecules stimulate prion protein conversion. Nature 2003; 425:717 - 720
- Deleault NR, Georghegan JC, Nishina K, Kascsak R, Williamson RA, Supattapone S. Protease-resistant prion protein amplification reconstituted with partially purified substrates and synthetic polyanions. J Biol Chem 2005; 280:26873 - 26879
- Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Nat Acad Sci 2007; 104:9741 - 9746
- Adler V, Zeiler B, Kryukov V, Kascsak R, Rubenstein R, Grossman A. Small, highly structured RNAs participate in the conversion of human recombinant PrP(Sen) to PrP(Res) in vitro. J Mol Biol 2003; 332:47 - 57
- Gomes MP, Millen TA, Ferreira PS, Cunha-e-Silva NL, Vieira TCRG, Almeida MS, Silva JL, Cordeiro Y. Prion Protein Complexed to N2a Cellular RNAs through Its N-terminal Domain Forms Aggregates and Is Toxic to Murine Neuroblastoma Cells. J Biol Chem 2008; 283:19616 - 19625
- Cordeiro Y, Kraineva J, Gomes MPB, Lopes MH, Martins VR, Lima LMTR, Foguel D, Winter R, Silva JL. The amino-terminal PrP domain is crucial to modulate prion misfolding and aggregation. Biophys J 2005; 89:2667 - 2676
- Dinger ME, Mercer TR, Mattick JS. RNAs as extracellular signaling molecules. J Mol Endocrinol 2008; 40:151 - 159
- Scott WG. Ribozymes. Curr Opin Struc Biol 2007; 17:280 - 286
- Napoli C, Lemieux C, Jorgensen R. Introduction of a Chimeric Chalcone Synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990; 2:279 - 289
- van der Krol AR, Mur LA, Beld M, Mol JN, Stuitje AR. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 1990; 2:291 - 299
- Smith HA, Swaney SL, Parks TD, Wernsman EA, Dougherty WG. Transgenic plant virus resistance mediated by untranslatable sense RNAs: expression, regulation and fate of nonessential RNAs. Plant Cell 1994; 6:1441 - 1453
- Palauqui JC, Elmayan T, Pollien JM, Vaucheret H. Systemic acquired silencing: transgenespecific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J 1997; 16:4738 - 4745
- Voinnet O, Baulcombe DC. Systemic signalling in gene silencing. Nature 1997; 389:553
- Garcia-Perez RD, Houdt HV, Depicker A. Spreading of posttranscriptional gene silencing along the target gene promotes systemic silencing. Plant J 2004; 38:594 - 602
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806 - 811
- Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 2005; 59:417 - 487
- St Laurent G III, Wahlestedt C. Noncoding RNAs: couplers of analog and digital information in nervous system function?. Trends Neurosci 2007; 30:612 - 621
- Mattick JS, Gagen MJ. The evolution of controlled multitasked gene networks: the role of introns and other noncoding RNAs in the development of complex organisms. Mol Biol Evol 2001; 18:1611 - 1630
- Mattick JS. A new paradigm for developmental biology. J Exp Biol 2007; 210:1526 - 1547
- Cordeiro Y, Kraineva J, Ravindra R, Lima LM, Gomes MP, Foguel D, Winter R, Silva JL. Hydration and packing effects on prion folding and beta-sheet conversion. High pressure spectroscopy and pressure perturbation calorimetry studies. J Biol Chem 2004; 279:32354 - 32359
- Tompa P, Csermely P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J 2004; 18:1169 - 1175
- Bera A, Roche AC, Nandi PK. Bending and unwinding of nucleic acid by prion protein. Biochemistry 2007; 46:1320 - 1328
- Leblanc P, Baas D, Darlix JL. Analysis of the interactions between HIV-1 and the cellular prion protein in a human cell line. J Mol Biol 2004; 337:1035 - 1051
- Lötscher M, Recher M, Lang KS, Navarini A, Hunziker L, Santimaria R, Glatzel M, Schwarz P, Böni J, Zinkernagel RM. Induced prion protein controls immune-activated retroviruses in the mouse spleen. PLoS ONE 2007; 2:1158