Abstract
The structure and the dissociation reaction of oligomers PrPoligo from reduced human prion huPrPC (23-231) have been studied by 1H-NMR and tryptophan fluorescence spectroscopy at varying pressure, along with circular dichroism and atomic force microscopy. The 1H-NMR and fluorescence spectral feature of the oligomer is consistent with the notion that the N-terminal residues including all seven Trp residues, are free and mobile, while residues 105~210, comprising the AGAAAAGA motif and S1-Loop-HelixA-Loop-S2-Loop-HelixC, are engaged in intra- and/or inter-molecular interactions. By increasing pressure to 200 MPa, the oligomers tend to dissociate into monomers which may be identified with PrPC*, a rare metastable form of PrPC stabilized at high pressure (Kachel et al. BMC. Struct. Biol. 6, 16). The results strongly suggest that the oligomeric form PrPoligo is in dynamic equilibrium with the monomeric forms via PrPC*, namely huPrPC ⇄ huPrPC* ⇄ huPrPoligo.
Introduction
A key step for the propagation of prion disease is a transformation of a-helical form of cellular prion protein PrPC to β-sheet rich, protease K-resistive form of prion known as PrPSc.Citation1,Citation2 Although a wealth of information has been accumulated for the basic structure of PrPC of different biological origin,Citation3,Citation4 little is known yet of the three dimensional structure of PrPSc except for a model from electron microscopy.Citation5
Recently, β-rich oligomers of limited length are shown to be a potent propagator of the protease K-resistive form of prion in vitro.Citation6 It has also been shown that the propagation of the oligomer form of prion between human and hamster (or mouse) takes place when Met residues at 138 and 139 of mouse or hamster is replaced by Ile.Citation7 These results indicate that structural information on oligomeric forms of prion is crucial to the understanding of the molecular mechanism of infectivity and the transformation mechanism of PrPC into PrPSc.
In the present work, human PrPC (huPrPC) is considered since it is structurally and functionally well studied. Besides the stable structure (PrPC) in solution, a meta-stable conformer (PrPC*) exists, as a putative precursor to PrPSc, in equilibrium with PrPC in human prion as well as in hamster prion as revealed from the high pressure NMR studies.Citation8–Citation10 In fact, high pressure NMR allowed to identify two structural substates of the ground state PrPC (N1 and N2) and of the excited state PrPC* (I1 and I2) simultaneously occurring at ambient pressure with the relative concentrations of 0.762, 0.226, 0.011, 0.0005, respectively. However, there has been little structural information on the oligomeric forms of prion, despite the increasing demand for it.
In the present study, human PrP oligomers (designated as huPrPoligo) are prepared from recombinant huPrPC(23–231) by reducing the single disulfide bond and treating the protein with denaturant. The formation of huPrPoligo(23–231) is monitored with circular dichroism (CD), atomic force microscopy (AFM) and tryptophan fluorescence. Direct 1H-NMR observation at variable pressure allowed to obtain some key information on the structure of the oligomer and its equilibrium with the monomeric form of human prion.
Results and Discussion
CD, AFM and fluorescence.
shows the CD spectrum of monomeric as well as oligomeric huPrP prepared as described in Materials and Methods. The CD spectrum of the monomeric protein corresponds closely to the known solution structure of the protein containing random-coil as well as a-helical and β-pleated sheet contributions. The oligomeric protein ( red) showed a typical β-sheet pattern, indicating that the a-rich form of human PrPC turned into the “β-rich form”. The CD-spectra a very similar to the spectra presented by Lührs et al.,Citation14 for native monomeric protein and an oligomeric form called PrPβ that was induced by heat treatment in the presence of phospholipids.
shows the AFM recordings of a sample of polymerized PrP. It exhibits many small round-shaped particles, showing the formation of oligomers without forming fibrils. The AFM figure shows on average an apparent diameter (horizontal) of ∼47 nm and an apparent height of ∼1.6 nm for a particle. To gain a rough estimate of the size of the huPrPoligo(23–231), we assume a flat cone with the aforementioned height and diameter and use the molecular weight of 28 kDa for huPrP(23–231) with a partial specific volume of 0.73, we get the average particle of 950 nm3 or ∼28 monomers. Tryptophan fluorescence spectra of oligomeric PrP were recorded at pressures varying in the range from 0.3 MPa to 335 MPa (). The maximum wavelength of emission is 352 nm at 0.3 MPa, suggesting that all Trp rings are exposed to the solvent. Increasing the pressure up to 400 MPa leaves the fluorescence intensity essentially unchanged and only leads to a small red shift of the fluorescence maximum, indicating that the environment of the tryptophan rings remains essentially unchanged.
1H-NMR spectrum of oligomeric huPrPC.
In spite of its high molecular mass, the 1H-NMR spectrum of oligomeric huPrPC(23–231) shows a number of well-resolved resonances (). At a first glance it is very similar to the spectrum of the monomeric protein (). The 15N enrichment of the latter protein leads to a doublet splitting of a resonance line from a proton directly bound to a nitrogen. This effect is especially well visible by the splitting of the resonances at approximately 10 ppm (center proton frequencies of 10.09, 10.12, 10.13 and 10.17 ppm). These signals correspond to the Hϵ1 of the seven tryptophan residues in PrP (Trp31, Trp57, Trp65, Trp73, Trp81, Trp89, Trp99). The amide signal of Phe141 is expected at 10.26 ppmCitation8 and is barely visible. Essentially the same pattern of Trp Hϵ1 resonances that is visible in the monomeric oxidized PrP can also be identified in the spectrum of the reduced, acid-treated PrP, but of course without the splitting due to 15N-coupling.
The spectrum of the reduced PrP is characterized by strong resonance lines clustered around random-coil frequencies with relatively little dispersion in individual resonances (). Compared to monomeric PrP, the lines are somewhat broader and only the part of the resonances that correspond to the unfolded, mobile N-terminus of monomeric PrP seems to be present. Incidentally, all seven tryptophan residues are located in the N-terminus of PrP and thus are markers for this region that can easily be recognized. Since they are clearly visible in the oligomeric reduced protein, the part of PrP containing the tryptophan residues must still have a very high internal mobility.
Immobilized residues in the oligomer huPrPoligo(23–231) should be characterized by very broad lines. Typical candidates for such behavior would be residues located in the well-folded C-terminal part of PrP if it remains folded in the oligomer. In line with this expectation, signals between 0.8 and 1 ppm, which have been assigned to protons of seventeen methyl groups from Val120, Val121, Ile138, Ile139, Val161, Val180, Ile184, Val189, Val203, Ile205, Val210 and four methylene groups from Met129, Leu130, Ile139, Ile182 in monomeric huPrPC(23–231),Citation4 is greatly reduced in . Furthermore, the well-resolved signals from -0.1 to 0.7 ppm in monomeric huPrPC(23–231) (signals 1–7 in ) diminish in the oligomer (). This would mean that these signals from residues 120 to 210, spanning S1-Loop-HelixA-Loop-S2-Loop-HelixC, are severely broadened and/or shifted to other regions of the spectrum. In accord with this, a peculiar broad component with a line width of about 200 Hz was observed at about 0.4 ppm in the oligomer (). Although the origin of this signal cannot be specified from the present study alone, the signal corresponds approximately to the mean chemical shift of the seven highfield shifted methyl signals of Leu125, Leu130, Ile139 and Ile182 in huPrPC(23–231)Citation4,Citation8 and it is likely that these residues are mainly responsible for this broad component.
In monomeric huPrPC(23–231), the methyl resonances of the six alanine residues produce a strong signal in the narrow range of 1.32 to 1.36 ppm.Citation4 Again this signal is significantly reduced after the polymerization (). It should not be completely suppressed, because protons of lysine and arginine residues of the presumably mobile N-terminal part resonate at the same position.Citation4 It is, therefore, likely that the segment containing the characteristic motif AGAAAAGA (residues 113–120) is also involved in the oligomerization. Lührs et al.,Citation14 showed that residues 113 to 120 are necessary for the formation of an oligomeric prion protein called PrPβ produced in the presence of lipids, whose CD spectrum is very similar to that of our huPrPoligo(23–231) shown in .
In conclusion, it is likely that, while the N-terminal residues including all seven Trp residues are free and exposed to the solvent, the C-terminal part (from residues 113 to 210 spanning the GAAAAGA motif and S1-Loop-HelixA-Loop-S2-Loop-HelixC are structured and engaged in intra- and/or inter-molecular interactions.
Pressure-jump 1H-NMR study of oligomeric huPrP.
Pressure-jump 1H-NMR was used previously to monitor the dissociation and association reaction of amyloid protofibrils.Citation13 We apply here the same technique to monitor the dissociation and association reaction of prion oligomers. and E show the 1H-NMR spectral change at 30 min and 46 h after a slow pressure-jump from 0.3 MPa to 200 MPa. The NMR signals for seven Trp ring NH show little changes upon the pressure-jump, which is consistent with the notion that residues 31–99 are not directly involved in the oligomerization. In contrast, some dramatic changes take place in other ranges of the spectrum. The most prominent effect is observed for the broad, highfield shifted signal around 0.4 ppm that was tentatively assigned to the methyl resonances of the folded C-terminal domain in the oligomeric state. The intensity of this signal is decreased and new relatively sharp signals appear at positions where signals are observed in monomeric huPrP(23–231) at 200 MPaCitation8 with some broadening. A simple interpretation for this effect is a pressure-induced depolymerization leading to a spectrum containing signals of oligomeric PrP together with dominantly monomeric PrP.
In line with this interpretation, the signals in the range from 0.8 to 1.0 ppm and those in the range of 1.32 to 1.36 ppm assigned to the AGAAAAGA motif partly reappear. In addition, the 1H spectrum appears to be more structured as to be expected from the reappearance of resonance lines from the monomeric protein. The spectral change is considered nearly finished within 30 min after the pressure is jumped to 200 MPa, because the spectrum after 46 h is virtually unchanged (), meaning that the system has reached an equilibrium in favor of monomeric species (). The rapid dissociation of the oligomeric PrP observed here is not surprising in view of the negative activation volume for the dissociation of amyloid protofibrilsCitation15 and the small size of the oligomers treated here.
A large fraction of the peculiar low pressure spectrum is restored within 30 min after the pressure-jump back from 200 MPa to 0.3 MPa (), showing that the oligomerization is a spontaneous process as observed previously in amyloid protofibril formation.Citation13 However, compared to the original spectrum (), the intensity of the broad component is still reduced and the signals assignable to monomeric huPrPC(23–231) have a higher intensity. This state prevails for a long time since the spectrum remains essentially unchanged for 9.5 hr (). Apparently, the reassociation process after pressure perturbation consists of two components, one relatively fast reaction with a time constant faster than 30 min and a second process with a time much larger than 9.5 h.
shows in detail the high-field part of the spectrum which exhibits a rather dramatic change upon pressure-jump to 200 MPa; the broad signal with a maximum at approximately 0.39 ppm (, †) diminishes its intensity and shifted upfield to approximately 0.50 ppm (, †*), while several new signals appear at 0.4∼0.9 ppm. Intriguingly, the signals at 0.4∼0.9 ppm at 200 MPa (, ‡*) coincide rather well with those of monomeric huPrPC(23–231)(non-reduced) measured at 200 MPa (, ‡).Citation8 Only the broad signal at −0.125 ppm arising from Leu130 Hδ1/δ2 and Ile139 Hγ2 is missing in and D, presumably due to extra broadening. In general, the high field part of the spectrum of the reduced PrP appears to contain two different components, a broad component assignable to the oligomeric PrP and sharper signals assignable to dominantly monomeric PrP. This is more obvious after reduction of the pressure to 0.3 MPa (, 7* and 8*) where the signals of Leu130 Hδ1/δ2 and Ile139 Hγ2 are observable at the same positions as in the oxidized monomer.
Reversible monomer-oligomer transition and its biological implication.
The reduction of the disulfide bond and the acid treatment of huPrPC(23–231) leads to an oligomeric protein PrPoligo with a CD spectrum typical for β-structures (), which is very similar to that of the oligomer called PrPβ prepared from non-reduced PrP in the presence of lipids.Citation14 After a pressure-jump to 200 MPa, PrPoligo partly dissociates into a semi-stable form of huPrPC in the time frame of 30 min. The resultant 1H-NMR spectrum () is very similar to that of the excited state I2 of non-reduced PrP described earlier ().Citation8,Citation10 A reduction of the pressure to 0.3 MPa leads to a spectrum () that differs from the original low pressure spectrum () in that it contains signals of monomeric PrP in a substantial amount, which are characteristic of the native states N1 and N2 described earlier.Citation8,Citation10 Even after 9.5 h its concentration has not changed, indicating that PrP in these native ground states (we collectively call huPrPC) is not directly involved in the formation of oligomers. At ambient pressure the excited states I1 and I2 (we collectively call huPrPC*) are quite rare with relative concentrations of 0.02 and 0.0005.Citation10
By combining the two equilibria, the minimum equilibrium scheme for the oligomerization is represented by
huPrPC ⇆ huPrPC* ⇆ huPrPoligo (Scheme 1).
Scheme 1 implies that the oligomerization reaction proceeds via the rare metastable conformers huPrPC* arising from the conformational fluctuation of huPrPC. Since the fluctuation huPrPC ⇆ huPrPC* is intrinsic to prion protein, the entire reactions represented by Scheme 1 should also take place in vivo, only with a low probability. The 1H-NMR and fluorescence spectral features of the oligomer are consistent with the notion that the N-terminal residues including all seven Trp residues (residues 31–99) are free and mobile, while residues 105∼210 in the C-terminal part, comprising the AGAAAAGA motif and S1-Loop-HelixA-Loop-S2-Loop-HelixC, are engaged in intra-and/or inter-molecular interactions. It is well known in huPrPSc that, while the C-terminal part is protease K-resistive, the N-terminal residues are selectively cleaved by protease K digestion.Citation16 Although the relationship of huPrPoligo to huPrPSc is not clear at present, the overall structure of huPrPoligo revealed in the present study seems to share a common feature with that of huPrPSc in that the N-terminal part is free while the C-terminal part is structured and is primarily responsible for association.
Materials and Methods
Preparation of oligomeric huPrP.
15N enriched human PrPC (huPrPC) (23–231) was a gift of K. Wüthrich. Unlabelled protein was prepared for oligomer studies by cultivating E. coli transformed with plasmids containing the cDNA for huPrPC. The preparation of β-form of huPrPC(23–231) followed the procedure of Jackson et al.;Citation11 the disulfide bond of purified huPrPC(23–231) was reduced by 100 mM dithiothreitol (DTT) in the presence of 6 M GdnHCl, 10 mM Na acetate and 10 mM tris-acetate (pH 8.0) for 16 hours. Then the protein solution was dialyzed against a buffer consisting of 10 mM sodium acetate and 10 mM tris-acetate (pH 4.0) for refolding in the presence of 1 mM DTT to prevent reoxidation. The final protein solution, prepared 5.4 mg/ml huPrPoligo (23–231) in 10 mM sodium acetate and 10 mM tris-acetate buffer containing 1 mM DTT (pH 4.0) was kept frozen until use.
CD, AFM and fluorescence measurements.
Circular dichroism spectra were recorded on a JASCO J-820 spectropolarimeter using a quartz cell of 0.1 mm light path at 298 K. Atomic force microscopic images were recorded on SPI-3800 (Seiko Instruments, Chiba) immediately after dilution of the 5.4 mg/ml solution of huPrPoligo and displaying on a fresh mica surface, Pressure-dependent tryptophan fluorescence spectra were measured at 298 K with the excitation wavelength at 295 nm on JASCO FP-6500 spectrofluororimeter equipped with a high-pressure chamber with sapphire windows (PCI-400) (Teramecs, Kyoto) connected to a hand-pump TP-500 (Teramecs, Kyoto) in the pressure range 0.3–400 MPa.
Pressure-jump1H-NMR spectroscopy.
One-dimensional (1D) 1H-NMR spectra were measured at 25°C on a Bruker AVANCE-500 spectrometer operating at 500 MHz equipped with a cryoprobe. The pressure-jump NMR measurements were performed by utilizing the on-line pressure cell techniqueCitation12 to measure the time-dependent 1H NMR spectra upon increasing pressure to 200 MPa and decreasing pressure to 0.3 MPa. Both Pressure-jumps were performed manually using a hand pump. Pressure was changed within approximately 1 min, after which five min was allowed for the equilibrium to be reached, as described previously.Citation13 Data were processed with XWIN NMR (Bruker) and NMR view.
Abbreviations
| huPrPC | = | the cellular form of human prion |
| PrPC* | = | a meta-stable conformer of PrPC |
| PrPSc | = | the scrapie form of prion |
| PrPoligo | = | the oligomeric form of prion, atomic force microscopy, CD, circular dichroism |
| NMR | = | nuclear magnetic resonance |
Figures and Tables
Figure 1 Circular dichroism spectra of monomeric oxidized huPrPC(23–231) (blue) and oligomeric reduced huPrPC(23–231) (red), recorded at 298 K. Monomeric oxidized huPrPC(23–231) (0.12 mM) was prepared in a buffer solution containing 10 mM sodium acetate and 10 mM tris-acetate, pH 8.0 and oligomeric reduced huPrPC(23–231) (1.9 mM) was prepared in a buffer solution containing 10 mM sodium acetate, 10 mM tris-acetate, pH 4.0.
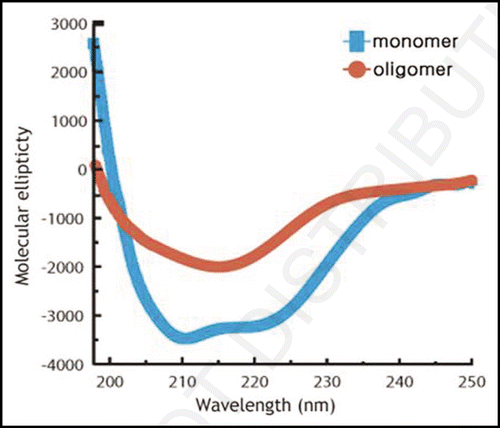
Figure 2 Atomic force microscopic image of oligomeric reduced huPrPC(23–231), recorded at room temperature.
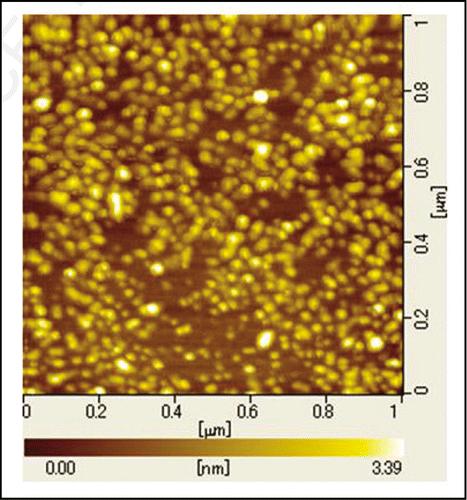
Figure 3 Trp fluorescence spectra of oligomeric reduced huPrPC(23–231) recorded at varying pressures and at 298 K.
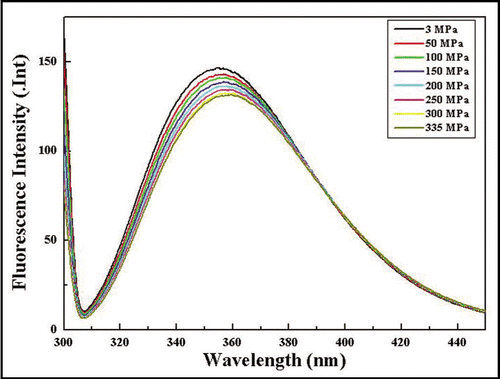
Figure 4 1H-NMR spectral changes of monomeric and oligomeric huPrPC(23–231) caused by pressure-jump up and down. (A) 1H NMR spectrum of (15N-enriched) monomeric huPrPC(23–231) at 293 K at 0.3 MPa. (B) The same as (A), but at 200 MPa. (C) 1H NMR spectrum of reduced oligomeric huPrPoligo(23–231) at 298 K at 0.3 MPa. (D) The same as (C), but 30 min after the pressure-jump from 0.3 MPa to 200 MPa. (E) The same as (C), but 46 h after the pressure-jump from 0.3 MPa to 200 MPa. (F) The same as (C), but 30 min after the pressure-jump back from 200 MPa to 0.3 MPa. (G) The same as (C), but 9.5 h after the pressure-jump back from 200 MPa to 0.3 MPa.
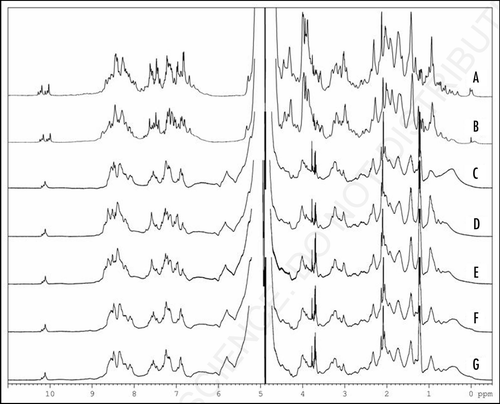
Figure 5 Oligomeric and monomeric of huPrPC 1H NMR recorded at varying pressures. (A) 1H NMR spectra of huPrPC(23–230) recorded at 293 K, pH 4.8 and at 0.1 MPa. Resonances of selected protons are labeled: (1) Leu130 Hδ2 (2) Leu125 Hδ2 (3) Ile139 Hδ1 (4) Ile182 Hδ1 (5) Ile182 Hγ2 (6) DSS (internal reference) (7) Leu130 Hδ1 (8) Ile139 Hγ2. (B) 1H NMR spectra of huPrPC(23–230) recorded at 293 K, pH 4.8 and at 200 MPa. [(A and B) data were reprinted from ref. Citation8]. (C) 1H NMR spectra of huPrPoligo(23–231) recorded at 298 K, pH 4.0 and at 0.3 MPa. (D) 1H NMR spectra of huPrPoligo(23–231) recorded at pressure jump up to 200 MPa after 46 h and at 298 K, pH 4.0. (E) 1H NMR spectra of huPrPoligo(23–231) recorded at pressure jump back to 0.3 MPa after 9.5 h and at 298 K, pH 4.0.
![Figure 5 Oligomeric and monomeric of huPrPC 1H NMR recorded at varying pressures. (A) 1H NMR spectra of huPrPC(23–230) recorded at 293 K, pH 4.8 and at 0.1 MPa. Resonances of selected protons are labeled: (1) Leu130 Hδ2 (2) Leu125 Hδ2 (3) Ile139 Hδ1 (4) Ile182 Hδ1 (5) Ile182 Hγ2 (6) DSS (internal reference) (7) Leu130 Hδ1 (8) Ile139 Hγ2. (B) 1H NMR spectra of huPrPC(23–230) recorded at 293 K, pH 4.8 and at 200 MPa. [(A and B) data were reprinted from ref. Citation8]. (C) 1H NMR spectra of huPrPoligo(23–231) recorded at 298 K, pH 4.0 and at 0.3 MPa. (D) 1H NMR spectra of huPrPoligo(23–231) recorded at pressure jump up to 200 MPa after 46 h and at 298 K, pH 4.0. (E) 1H NMR spectra of huPrPoligo(23–231) recorded at pressure jump back to 0.3 MPa after 9.5 h and at 298 K, pH 4.0.](/cms/asset/de1a58f6-03f7-4018-b5bf-7a6e6300b5b0/kprn_a_10907148_f0005.gif)
Acknowledgements
This work was supported by the BSE Control Project from MAFF (the Ministry of Agriculture, Forestry and Fisheries of Japan) and by a Grant-in-Aid for Scientific Research No. 16370054 from MEXT (the Ministry of Education, Culture, Sports, Science and Technology of Japan), and carried out under the sponsorship of an Academic Frontier Program of MEXT. The German-Japanese cooperation was supported by the DFG.
References
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, et al. Conversion of α-Helices into β-Sheets Features in the Formation of the Scrapie Prion Proteins. Proc Natl Acad Sci USA 1993; 90:10962 - 10966
- Bocharova OV, Breydo L, Parfenov AS, Salnikov VV, Baskakov IV. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrP(Sc). J Mol Biol 2005; 346:645 - 659
- James TL, Liu H, Ulyanov NB, Farr-Jones S, Zhang H, Donne DG, et al. Solution structure of a 142-residue recombinant prion protein corresponding to the infectious fragment of the scrapie isoform. Proc Natl Acad Sci USA 1997; 94:10086 - 10091
- Zahn R, Liu A, Luhrs T, Riek R, von Schroetter C, Lopez GF, et al. NMR solution structure of the human prion protein. Proc Natl Acad Sci USA 2000; 97:145 - 150
- Wille H, Michelitsch MD, Guenebaut V, Supattapone S, Serban A, Cohen FE, et al. Structural studies of the scrapie prion protein by electron crystallography. Proc Natl Acad Sci USA 2002; 99:3563 - 3568
- Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, et al. The most infectious prion protein particles. Nature 2005; 437:257 - 261
- Vanik DL, Surewicz KA, Surewicz WK. Molecular Basis of Barriers for Interspecies Transmissibility of Mammalian Prions. Mol Cell 2004; 14:139 - 145
- Kachel N, Kremer W, Zahn R, Kalbitzer HR. Observation of intermediate states of the human prion protein by high pressure NMR spectroscopy. BMC Structural Biology 2006; 6:16
- Kuwata K, Li H, Yamada H, Legname G, Prusiner SB, Akasaka K, et al. Locally disordered conformer of the hamster prion protein: a crucial intermediate to PrPSc?. Biochemistry 2002; 41:12277 - 12283
- Kremer W, Kachel N, Kuwata K, Akasaka K, Kalbitzer HR. Species-specific differences in the intermediate states of human and Syrian Hamster prion protein detected by high pressure NMR spectroscopy. J Biol Chem 2007; 282:22689 - 22698
- Jackson GS, Hosszu LL, Power A, Hill AF, Kenney J, Saibil H, et al. Reversible conversion of monomeric human prion protein between native and fibrilogenic conformations. Science 1999; 283:1935 - 1937
- Akasaka K, Yamada H. On-Line Cell High Pressure Nuclear Magnetic Resonance Technique: Application to Protein Studies. Methods in Enzymology 2001; 338:134 - 158
- Kamatari YO, Yokoyama S, Tachibana H, Akasaka K. Pressure-jump NMR Study of Dissociation and Association of Amyloid Protofibrils. J Mol Biol 2005; 349:916 - 921
- Lührs T, Zahn R, Wüthrich K. Amyloid formation by recombinant full-length prion proteins in phospholipid bicelle solutions. J Mol Biol 2006; 357:833 - 841
- Abdul Latif AR, Kono R, Tachibana H, Akasaka K. Pressure dissociation kinetics of amyloid protofibrils. Biophys J 2007; 92:323 - 329
- Parchi P, Zou W, Wang W, Brown P, Capellari S, Ghetti B, et al. Genetic influence on the structural variations of the abnormal prion protein. Proc Natl Acad Sci USA 2000; 97:10168 - 10172