Abstract
PrPC is associated with a variety of functions, and its ability to interact with a multitude of partners, including itself, may largely explain PrP multifunctionality and the lack of consensus on the genuine physiological function of the protein in vivo. In contrast, there is a consensus in the literature that alterations in PrPC trafficking and intracellular retention result in neuronal degeneration. In addition, a proteolytic modification in the late secretory pathway termed the α-cleavage induces the secretion of PrPN1, a PrPC-derived metabolite with fascinating neuroprotective activity against toxic oligomeric Aβ molecules implicated in Alzheimer disease. Thus, studies focusing on understanding the regulation of PrPC trafficking to the cell surface and the modulation of α-cleavage are essential. The objective of this commentary is to highlight recent evidences that PrPC homodimerization stimulates trafficking of the protein to the cell surface and results in high levels of PrPN1 secretion. We also discuss a hypothetical model for these results and comment on future challenges and opportunities.
PrP Homodimerization
PrP dimers were experimentally detected in bovine, mouse and hamster brain homogenates,Citation1-Citation3 and in N2a cells expressing hamster PrPC or endogenous PrPC 4, 5. Predictions and experimental evidences clearly showed that the hydrophobic domain (residues 112-MAGAAAAGAVVGGLGGYMLGSA-133) mediates PrPC dimerization.Citation5,Citation6
In the context of prion propagation, PrPC dimerization is generally perceived as an important molecular step toward conformational change of the soluble α-helical PrPC to the insoluble β-sheet-rich PrPSc 7–10. Also, the connection between dimerization and toxicity has been supported for several years by a model suggesting in vivo noxiousness of cross-linked PrPC at neurons surface.Citation11 However, this model was recently clearly invalidated, and passive immunotherapy with monoclonal antibodies against PrPC is seriously considered.Citation12-Citation14
Results from two different studies led to the conclusion that dimerization of PrPC is actually a positive mechanism in physiological conditions. First, in experimental settings where PrPC displays a stress-protective signaling activity, dimerization correlates with neuroprotection.Citation5 In contrast, impaired dimerization in prion-infected cells correlates with exacerbated sensitivity to stress. In a second approach, we used an inducible dimerization strategy to control PrPC dimerization in cell culture. Using a permeable dimerizer, we discovered that PrPC dimerization increases PrPC trafficking to the cell surface. This resulted in a very large increase of all extracellular PrPC species, including PrPN1 and shed PrPC (), within a few hours.Citation15 In this experiment, the burst in secretion of PrPN1 after PrPC dimerization resulted in levels sufficient to inhibit Aβ oligomers-mediated cell death compared with basal levels of PrPN1 that were clearly inadequate.Citation15 In this experimental setting, dimerization is a molecular switch between cell death and survival. Enforced dimerization of PrPC also resulted in large increase of PrPC1 and shed PrPC 15. Other studies demonstrated that PrPC1 acts as a GPI-anchored dominant negative inhibitor of PrPSc formation,Citation16 and that soluble dimeric PrP binds PrPSc and can antagonize prion propagation.Citation17 Thus, increased trafficking of PrPC may be an effective mechanism to deliver protective molecules at the cell surface and in the extracellular space.
Figure 1. Enforced dimerization increased shedding of PrPC. Left panel, western blot analysis of PrPC immunoprecipitated from the cultured medium of cells expressing Fv-PrP, without (control) or with enforced dimerization (dimerizer). Right panel, Densitometric analysis of four independent experiments showed that addition of the dimerizer caused a 3.6 fold increase of shed PrPC compared with control cells. In this experiment, the dimerizer binds to the Fv domain.Citation15
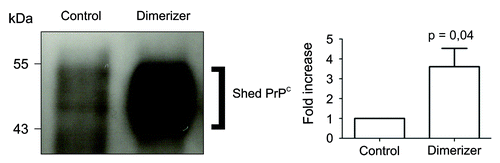
Overall, these studies strongly support the proposition that physiological PrPC dimers are neuroprotective.
Mechanism and Regulation of Dimerization-Induced PrPC Cell Surface Delivery
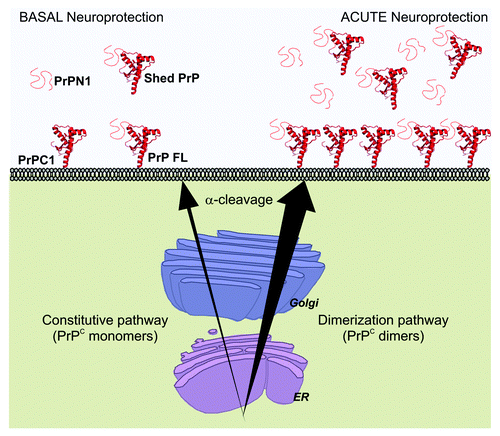
We did not elucidate the detailed mechanism of how enforced dimerization stimulates PrPC delivery at the plasma membrane. However, our observations provided insights for two characteristics. First, this mechanism is membrane anchor-independent. Thus, the interaction with a membrane partner or the localization in lipid rafts is not essential for dimerization-induced trafficking. Second, levels of total PrP species after dimerization were noticeably increased after dimerization.Citation15 Since dimerization did not change PrPC stability (), we hypothesize that it improves maturation efficiency and passage through protein folding quality control checkpoints. A third observation from previous reports indicates that deletion of the natural dimerization domain does not prevent cell membrane delivery of the protein.Citation18 From these data, we propose a model in which two secretion mechanisms regulate PrPC trafficking to the cell surface (). A constitutive delivery mechanism independent from the presence of the dimerization domain controls basal levels of PrPC at the plasma membrane. In addition, a dimerization-regulated secretion mechanism allows very large increases of extracellular PrPC species in a short period of time. Compared with the constitutive pathway, the efficiency of the regulated pathway is remarkable. By definition, our model is hypothetical, and the use of an artificial dimerization strategy to enforce PrPC homodimerization may have exaggerated a normally occurring mechanism. Thus, the large increase in PrPC trafficking after dimerization may not be as dramatic in physiological conditions when the natural hydrophobic domain mediates PrPC dimerization.
Figure 2. Enforced dimerization does not increase the stability of PrPC dimers. Fv-PrPC expressing cells were treated with vehicle (control) or the dimerizer for 20 h. Cells were subsequently treated with cycloheximide (0h) and total cellular Fv-PrPC levels were estimated by densitometric analyses over time. Levels of Fv-PrPC at 8 and 24 h post-treatment were normalized to 0h. Dimerization did not significantly change the degradation rate of Fv-PrPC. For experimental details, see Béland et al.Citation15
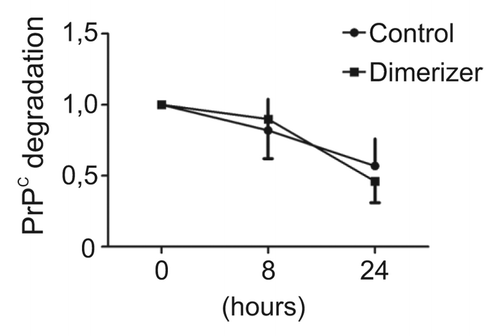
Figure 3. Acute neuroprotection induced by dimerization of PrPC. Constitutive secretion of PrPC provides cells with basal levels of neuroprotective species (Left). After dimerization, PrPC secretion increases sharply and causes a strong rise of full length PrPC (FL) and PrPC1 at the cell membrane and of PrPN1 and shed PrPC in the extracellular medium. PrPN1 and shed PrPC are able to bind and neutralize toxic β-sheet oligomers (Right) and provide a strong neuroprotective response. More efficient trafficking of PrPC to the cell surface also suggests that the risk of potential intracellular accumulation of toxic PrPC species is reduced.
That PrPC may have evolved a well conserved domain to stimulate the efficiency of its own trafficking through the secretory pathway is possible. Indeed, PrPC signal sequence is slightly inefficient and acute stress in the endoplasmic reticulum results in intracellular accumulation and neuronal toxicity in cultured cells and in vivo.Citation19-Citation21 In addition, PrPC familial or artificial mutants that accumulate intracellularly are also neurotoxic.Citation22,Citation23 Dimerization may help preventing fatal accumulation of intracellular PrPC.
According to our model, we predict that promoting PrPC dimerization in the secretory pathway may help fighting neurotoxic insults, including the neutralisation of β-sheet oligomers (). In order to modulate PrPC dimerization as a possible therapeutic intervention, an important aim is to elucidate its regulation. It is tempting to speculate that endogenous molecules may bind to the dimerization domain and modulate the dimerization process. This domain binds several ligands, including lipids, heparin sulfate glycosaminoglycans, the secreted glycoprotein vitronectin and the secreted heat shock protein Sti1.Citation24 However, these mostly extracellular ligands are unlikely to modulate PrPC dimerization in the secretory pathway. Currently, the modulation of endogenous PrPC dimerization is not possible and finding a strategy to stimulate this mechanism remains an important challenge. Interestingly, the model that dimerization improves the efficiency of delivery at the cell surface is reminiscent of other membrane receptors, including some GPCR proteins.Citation25-Citation29 In this field, targeting molecular chaperones that regulate GPCRs dimerization and trafficking is a clear novel therapeutic avenue.Citation29
The Elusive Connection between Dimerization, α-Cleavage and Membrane Topology
During translocation into the ER, 80–90% of the total PrPC chains pass completely the ER membrane and get into the lumen.Citation19,Citation30 Additionally, 2% of total PrPC inserts either with their N terminus in the ER lumen (NtmPrP) or in the opposite orientation (CtmPrP).Citation21 In these type I and type II single-pass transmembrane forms, respectively, the dimerization domain acts as a transmembrane domain. Experimental mutations that increase the transmembrane domain hydrophobicity result in higher levels of CtmPrP and increased neurodegeneration in mice.Citation31-Citation33 Interestingly, five disease-associated mutations in human PRNP (Pro105Leu, Gly114Val, Ala117Val, Gly131Val and Ala133Val) increase the hydrophobicity of the transmembrane domain. Thus, CtmPrP may be the neurotoxic molecule in the corresponding familial forms of human prion disease.Citation34
Several artificial deletions of different size in this domain outside of the α-cleavage site partially or completely inhibit the α-cleavage,Citation35-Citation37 indicating that the dimerization domain is also essential for this cleavage. Since α-cleavage occurs in the late secretory pathway,Citation38 PrPC mutants unable to reach the cell surface should be resistant to α-cleavage. However, some mutants with deletions that do not affect the α-cleavage site in the dimerization domain-still traffic to the plasma membrane but have a reduced α-cleavage activity.Citation36 These results indicate that the dimerization domain is a key feature recognized by the enzyme responsible for this cleavage.
It is certainly possible that the same domain is involved in dimerization, α-cleavage and membrane integration of minor topological forms, three apparently independent functions. PrPC is a multifunctional protein with multiple topological forms which interacts with a large variety of ligands. Thus, a PrPC domain of 22 amino acids able to display these three functions and also bind different substrates would not be surprising for researchers in the prion field. Alternatively, these three functions may be connected if dimerization were a requirement for α-cleavage. This issue will likely require the identification of the α-protease in order to be appropriately addressed.
PrPC-Based Therapeutic Opportunities for Neurodegenerative Disorders
Levels of secreted PrPN1 achieved after dimerization are impressive.Citation15 This burst of secretion is particularly interesting since PrPN1 neutralizes β-sheet-rich oligomers toxicity, including soluble Aβ oligomers.Citation15,Citation39,Citation40 Specifically, Alzheimer disease is set to surge in the next future, and any potential therapeutic strategy disserves in depth investigations. In prion diseases, the N-terminal polybasic residues of PrPC bind PrPSc and are required for efficient prion propagation.Citation41 Increasing extracellular PrPN1 levels would be expected to prevent PrPSc binding to the neuronal surface and inhibit prion conversion and induction of intracellular toxic signals. However, several issues should be addressed prior to envisaging a therapeutic avenue based on PrPN1. First, biochemical characterizations of PrPN1 is lacking such as whether it is secreted as monomeric or oligomeric species and in association with other proteins. Second, the mechanism of how PrPN1 neutralizes β-sheet-rich oligomers remains to be determined. A direct interaction is the most straightforward hypothesis, but this interaction has not been validated yet in vivo. Third, it will be important to determine the fate of neutralized β-sheet-rich oligomers complexes and the mechanism of clearance of these fatal species. Last but not least, provided that all previous issues are resolved and the bioactivity of PrPN1 against β-sheet-rich oligomers is well characterized, the delivery mode of PrPN1 through increased dimerization or direct administration will also have to be addressed. Manipulating PrPC trafficking or using a PrPC metabolite as a possible therapeutic molecule may sound peculiar. Yet, there is a large body of evidence that PrPC is neuroprotective,Citation5,Citation42-Citation44 and the translation of this characteristic in medical settings in the future might not be science fiction.Citation45
Acknowledgments
This research was supported by a grant from the Canadian Institutes for Health Research. We thank Julie Motard for critical reading of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Bendheim PE, Bolton DC. A 54-kDa normal cellular protein may be the precursor of the scrapie agent protease-resistant protein. Proc Natl Acad Sci U S A 1986; 83:2214 - 8; http://dx.doi.org/10.1073/pnas.83.7.2214; PMID: 2421288
- Meyer RK, Lustig A, Oesch B, Fatzer R, Zurbriggen A, Vandevelde M. A monomer-dimer equilibrium of a cellular prion protein (PrPC) not observed with recombinant PrP. J Biol Chem 2000; 275:38081 - 7; http://dx.doi.org/10.1074/jbc.M007114200; PMID: 10967124
- Sklaviadis TK, Manuelidis L, Manuelidis EE. Physical properties of the Creutzfeldt-Jakob disease agent. J Virol 1989; 63:1212 - 22; PMID: 2492609
- Priola SA, Caughey B, Wehrly K, Chesebro B. A 60-kDa prion protein (PrP) with properties of both the normal and scrapie-associated forms of PrP. J Biol Chem 1995; 270:3299 - 305; http://dx.doi.org/10.1074/jbc.270.7.3299; PMID: 7852415
- Rambold AS, Müller V, Ron U, Ben-Tal N, Winklhofer KF, Tatzelt J. Stress-protective signalling of prion protein is corrupted by scrapie prions. EMBO J 2008; 27:1974 - 84; http://dx.doi.org/10.1038/emboj.2008.122; PMID: 18566584
- Warwicker J. Modeling a prion protein dimer: predictions for fibril formation. Biochem Biophys Res Commun 2000; 278:646 - 52; http://dx.doi.org/10.1006/bbrc.2000.3829; PMID: 11095963
- Tompa P, Tusnády GE, Friedrich P, Simon I. The role of dimerization in prion replication. Biophys J 2002; 82:1711 - 8; http://dx.doi.org/10.1016/S0006-3495(02)75523-9; PMID: 11916832
- Stöhr J, Weinmann N, Wille H, Kaimann T, Nagel-Steger L, Birkmann E, et al. Mechanisms of prion protein assembly into amyloid. Proc Natl Acad Sci U S A 2008; 105:2409 - 14; http://dx.doi.org/10.1073/pnas.0712036105; PMID: 18268326
- Jansen K, Schäfer O, Birkmann E, Post K, Serban H, Prusiner SB, et al. Structural intermediates in the putative pathway from the cellular prion protein to the pathogenic form. Biol Chem 2001; 382:683 - 91; http://dx.doi.org/10.1515/BC.2001.081; PMID: 11405232
- Goggin K, Bissonnette C, Grenier C, Volkov L, Roucou X. Aggregation of cellular prion protein is initiated by proximity-induced dimerization. J Neurochem 2007; 102:1195 - 205; http://dx.doi.org/10.1111/j.1471-4159.2007.04611.x; PMID: 17663754
- Solforosi L, Criado JR, McGavern DB, Wirz S, Sánchez-Alavez M, Sugama S, et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 2004; 303:1514 - 6; http://dx.doi.org/10.1126/science.1094273; PMID: 14752167
- Li L, Napper S, Cashman NR. Immunotherapy for prion diseases: opportunities and obstacles. Immunotherapy 2010; 2:269 - 82; http://dx.doi.org/10.2217/imt.10.3; PMID: 20635933
- Klöhn PC, Farmer M, Linehan JM, O’Malley C, Fernandez de Marco M, Taylor W, et al. PrP antibodies do not trigger mouse hippocampal neuron apoptosis. Science 2012; 335:52; http://dx.doi.org/10.1126/science.1215579; PMID: 22223800
- Aguzzi A, O’Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov 2010; 9:237 - 48; http://dx.doi.org/10.1038/nrd3050; PMID: 20190788
- Béland M, Motard J, Barbarin A, Roucou X. PrP(C) homodimerization stimulates the production of PrPC cleaved fragments PrPN1 and PrPC1. J Neurosci 2012; 32:13255 - 63; http://dx.doi.org/10.1523/JNEUROSCI.2236-12.2012; PMID: 22993441
- Westergard L, Turnbaugh JA, Harris DA. A naturally occurring C-terminal fragment of the prion protein (PrP) delays disease and acts as a dominant-negative inhibitor of PrPSc formation. J Biol Chem 2011; 286:44234 - 42; http://dx.doi.org/10.1074/jbc.M111.286195; PMID: 22025612
- Meier P, Genoud N, Prinz M, Maissen M, Rülicke T, Zurbriggen A, et al. Soluble dimeric prion protein binds PrP(Sc) in vivo and antagonizes prion disease. Cell 2003; 113:49 - 60; http://dx.doi.org/10.1016/S0092-8674(03)00201-0; PMID: 12679034
- Winklhofer KF, Heske J, Heller U, Reintjes A, Muranyi W, Moarefi I, et al. Determinants of the in vivo folding of the prion protein. A bipartite function of helix 1 in folding and aggregation. J Biol Chem 2003; 278:14961 - 70; http://dx.doi.org/10.1074/jbc.M209942200; PMID: 12556465
- Rane NS, Yonkovich JL, Hegde RS. Protection from cytosolic prion protein toxicity by modulation of protein translocation. EMBO J 2004; 23:4550 - 9; http://dx.doi.org/10.1038/sj.emboj.7600462; PMID: 15526034
- Rane NS, Kang SW, Chakrabarti O, Feigenbaum L, Hegde RS. Reduced translocation of nascent prion protein during ER stress contributes to neurodegeneration. Dev Cell 2008; 15:359 - 70; http://dx.doi.org/10.1016/j.devcel.2008.06.015; PMID: 18804434
- Rane NS, Chakrabarti O, Feigenbaum L, Hegde RS. Signal sequence insufficiency contributes to neurodegeneration caused by transmembrane prion protein. J Cell Biol 2010; 188:515 - 26; http://dx.doi.org/10.1083/jcb.200911115; PMID: 20156965
- Ma J, Wollmann R, Lindquist S. Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science 2002; 298:1781 - 5; http://dx.doi.org/10.1126/science.1073725; PMID: 12386337
- Ashok A, Hegde RS. Selective processing and metabolism of disease-causing mutant prion proteins. PLoS Pathog 2009; 5:e1000479; http://dx.doi.org/10.1371/journal.ppat.1000479; PMID: 19543376
- Beland M, Roucou X. The prion protein unstructured N-terminal region is a broad-spectrum molecular sensor with diverse and contrasting potential functions. J Neurochem 2012; 120:853-68; PMID: 22145935; DOI: 10.1111/j.1471-4159.2011.07613.x; 10.1111/j.1471-4159.2011.07613.x.
- Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 2002; 42:409 - 35; http://dx.doi.org/10.1146/annurev.pharmtox.42.091701.082314; PMID: 11807178
- Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep 2004; 5:30 - 4; http://dx.doi.org/10.1038/sj.embor.7400052; PMID: 14710183
- Milligan G. The role of dimerisation in the cellular trafficking of G-protein-coupled receptors. Curr Opin Pharmacol 2010; 10:23 - 9; http://dx.doi.org/10.1016/j.coph.2009.09.010; PMID: 19850521
- Kobayashi H, Ogawa K, Yao R, Lichtarge O, Bouvier M. Functional rescue of beta-adrenoceptor dimerization and trafficking by pharmacological chaperones. Traffic 2009; 10:1019-33; PMID: 19515093; DOI: 10.1111/j.1600-0854.2009.00932.x; 10.1111/j.1600-0854.2009.00932.x.
- Williams D, Devi LA. Escorts take the lead molecular chaperones as therapeutic targets. Prog Mol Biol Transl Sci 2010; 91:121 - 49; http://dx.doi.org/10.1016/S1877-1173(10)91005-3; PMID: 20691961
- Yedidia Y, Horonchik L, Tzaban S, Yanai A, Taraboulos A. Proteasomes and ubiquitin are involved in the turnover of the wild-type prion protein. EMBO J 2001; 20:5383 - 91; http://dx.doi.org/10.1093/emboj/20.19.5383; PMID: 11574470
- Stewart RS, Piccardo P, Ghetti B, Harris DA. Neurodegenerative illness in transgenic mice expressing a transmembrane form of the prion protein. J Neurosci 2005; 25:3469 - 77; http://dx.doi.org/10.1523/JNEUROSCI.0105-05.2005; PMID: 15800202
- Hegde RS, Mastrianni JA, Scott MR, DeFea KA, Tremblay P, Torchia M, et al. A transmembrane form of the prion protein in neurodegenerative disease. Science 1998; 279:827 - 34; http://dx.doi.org/10.1126/science.279.5352.827; PMID: 9452375
- Hegde RS, Tremblay P, Groth D, DeArmond SJ, Prusiner SB, Lingappa VR. Transmissible and genetic prion diseases share a common pathway of neurodegeneration. Nature 1999; 402:822 - 6; http://dx.doi.org/10.1038/45574; PMID: 10617204
- Chakrabarti O, Ashok A, Hegde RS. Prion protein biosynthesis and its emerging role in neurodegeneration. Trends Biochem Sci 2009; 34:287 - 95; http://dx.doi.org/10.1016/j.tibs.2009.03.001; PMID: 19447626
- Wik L, Klingeborn M, Willander H, Linne T. Separate mechanisms act concurrently to shed and release the prion protein from the cell. Prion 2012; 6:498-509; PMID: 23093798; DOI: 10.4161/pri.22588; 10.4161/pri.22588.
- Oliveira-Martins JB, Yusa S, Calella AM, Bridel C, Baumann F, Dametto P, et al. Unexpected tolerance of alpha-cleavage of the prion protein to sequence variations. PLoS One 2010; 5:e9107; http://dx.doi.org/10.1371/journal.pone.0009107; PMID: 20161712
- Bremer J, Baumann F, Tiberi C, Wessig C, Fischer H, Schwarz P, et al. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci 2010; 13:310 - 8; http://dx.doi.org/10.1038/nn.2483; PMID: 20098419
- Walmsley AR, Watt NT, Taylor DR, Perera WS, Hooper NM. alpha-cleavage of the prion protein occurs in a late compartment of the secretory pathway and is independent of lipid rafts. Mol Cell Neurosci 2009; 40:242 - 8; http://dx.doi.org/10.1016/j.mcn.2008.10.012; PMID: 19056496
- Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Müller V, Krishnan R, et al. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J 2011; 30:2057 - 70; http://dx.doi.org/10.1038/emboj.2011.86; PMID: 21441896
- Guillot-Sestier MV, Sunyach C, Ferreira ST, Marzolo MP, Bauer C, Thevenet A, et al. α-Secretase-derived fragment of cellular prion, N1, protects against monomeric and oligomeric amyloid β (Aβ)-associated cell death. J Biol Chem 2012; 287:5021 - 32; http://dx.doi.org/10.1074/jbc.M111.323626; PMID: 22184125
- Turnbaugh JA, Unterberger U, Saá P, Massignan T, Fluharty BR, Bowman FP, et al. The N-terminal, polybasic region of PrP(C) dictates the efficiency of prion propagation by binding to PrP(Sc). J Neurosci 2012; 32:8817 - 30; http://dx.doi.org/10.1523/JNEUROSCI.1103-12.2012; PMID: 22745483
- Lo RY, Shyu WC, Lin SZ, Wang HJ, Chen SS, Li H. New molecular insights into cellular survival and stress responses: neuroprotective role of cellular prion protein (PrPC). Mol Neurobiol 2007; 35:236 - 44; http://dx.doi.org/10.1007/s12035-007-8003-y; PMID: 17917112
- Roucou X, Giannopoulos PN, Zhang Y, Jodoin J, Goodyer CG, LeBlanc A. Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells. Cell Death Differ 2005; 12:783 - 95; http://dx.doi.org/10.1038/sj.cdd.4401629; PMID: 15846375
- Rial D, Piermartiri TC, Duarte FS, Tasca CI, Walz R, Prediger RD. Overexpression of cellular prion protein (PrP(C)) prevents cognitive dysfunction and apoptotic neuronal cell death induced by amyloid-beta (abeta(1)(-)(4)(0)) administration in mice. Neuroscience 2012; 215:79-89; PMID: 22537845; DOI: 10.1016/j.neuroscience.2012.04.034; 10.1016/j.neuroscience.2012.04.034.
- Biasini E, Harris DA. Targeting the cellular prion protein to treat neurodegeneration. Future Med Chem 2012; 4:1655-8; PMID: 22924502; DOI: 10.4155/fmc.12.114; 10.4155/fmc.12.114.