Abstract
Since the appearance in 1986 of epidemic of bovine spongiform encephalopathy (BSE), a new form of neurological disease in cattle which also affected human beings, many diagnostic and research activities have been performed to develop detection and therapeutic tools. A lot of progress was made in better identifying, understanding and controlling the spread of the disease by appropriate monitoring and control programs in European countries. This paper reviews the recent knowledge on pathogenesis, transmission and persistence outside the host of prion, the causative agent of transmissible spongiform encephalopathies (TSE) in mammals with a particular focus on risk (re)assessment and management of biosafety measures to be implemented in diagnostic and research laboratories in Belgium. Also, in response to the need of an increasing number of European diagnostic laboratories stopping TSE diagnosis due to a decreasing number of TSE cases reported in the last years, decontamination procedures and a protocol for decommissioning TSE diagnostic laboratories is proposed.
Introduction
Transmissible spongiform encephalopathy (TSE) is a family of neurodegenerative diseases found in several animal species including human (). Among these, the bovine spongiform encephalopathy (BSE) came first to the attention of the agricultural and scientific community in 1986 with the appearance of a new form of neurological disease in cattle in the United Kingdom. The origin of the BSE disease was a mystery but it propagated rapidly in the UK and later in different countries around the world. The rapid spread was linked to feeding to cattle, sheep, and goats meat and bone meat (MBM) contaminated with high risk tissues from BSE affected cattle.Citation1,Citation2 Ten years after the beginning of BSE crisis, commonly named “mad cow disease crisis,” a new form or variant of Creutzfeldt-Jacob disease (vCJD) emerged in human which was causally linked to BSE.Citation2-Citation4 In January 2012, a total of 176 cases of vCJD were reported in the UK and although to a lesser extent cases were described in other countries.
Table 1. Human and animal prion diseases, their acronyms and their probable etiology
The mad cow disease crisis represented an important issue for public health as well as for trade involving movements of animals. Safety measures and rules to control and prevent the transmission of the disease by feed and food were adopted first in the UK and subsequently in the European Union. These measures included 3 major action lines: the removal of Specified Risk Material (SRM), the ban on feeding of MBM and processed animal protein, and the establishment of monitoring programs.Citation5,Citation6 A European framework of surveillance of TSE in cattle, sheep, and goat was established which allowed to determine the risk status of EU member states on a regular time frame and to follow the evolution of TSE prevalence over the years. In parallel, a European Creutzfeldt-Jacob Disease Surveillance Network (EuroCJD and later on NeuroCJD) which encompasses centers in European and non-European countries (Australia, Japan, Canada) allows data comparison for all CJDs since 1993 with the objective to further develop the surveillance of vCJD and to identify novel forms of CJD. The recent surveillance framework highlights that the number of BSE cases has decreased in Europe in the last years (). Since 2009, 17 European Member States have even been authorized to review their monitoring programs, based on their favorable epidemiological situation, leading to a diminution of roughly 30% of the number of tests performed annually in the EU in 2009 compared with 2008 (Chart 1 in Annex III to CSWD).Citation7 During the 80th General session in May 2012, the OIE decided to put Belgium in the category of countries with negligible BSE risk, which resulted together with the EFSA publication on the minimal sample size, to the stop of control of healthy slaughtered cattle in Belgium since the beginning of 2013.Citation8
Table 2. Number of reported cases of bovine spongiform encephalopathy in farmed cattle worldwide
Since the mad cow disease crisis and the emergence of vCJD in humans, considerable research efforts are made in an attempt to establish the TSE pathogenesis and develop detection and therapeutic tools for humans and animals. In Europe, R&D laboratories worked intensively to develop rapid, sensitive and specific diagnostic testing of TSEs in livestock as well as in human in order to stop the transmission of the disease. In the first years of the crisis, the number of facilities handling TSE agents increased as a function of the monitoring needs. In several European member states such as Belgium these laboratory activities fall under the scope of regulations on the protection of workers from risks related to exposure to biological agents at work (implementing EU Directive 2000/54/EC) and on the contained use of genetically modified microorganisms (GMM) and/or pathogenic microorganisms (implementing Directive 2009/41/EC).Citation9,Citation10 The latter foresees that a contained use of GMM and/or pathogenic microorganism is subject to a risk assessment to define proper risk management including the adoption of adequate containment measures and work practices. The aim is to provide a high level of safety for the general population and the environment. The risk assessment is based on the identification of potentially harmful properties of the TSE causing agents such as pathogenicity, transmission mode, persistence and stability of the agent in the environment and availability of effective prophylaxis or therapy.
Today, in several EU member states the epidemiologic situation has evolved to a significant decrease in TSEs cases with a concomitant decrease in TSE detection testing. As a result TSE diagnostic laboratories see a reduction of their main activity and competent authorities decided to limit this activity to a lower number of laboratories. What will be the future of these laboratories that have performed rapid testing on animal samples during years? Should they now undertake another type of activity or should they simply stop TSE diagnostic activity and close down? In either case, the first step they must face will concern the decommissioning of the facility. In other words, facility should be properly decontaminated using specific and rigorous methods adapted to TSE causing agents and then dismantled.
This paper reviews the state-of-the-art of knowledge on TSE in animals and human with a focus on the risk assessment of diagnostic activities and scientific research handling TSE causative agents. Decontamination procedures and decommissioning of these facilities are also reviewed in an attempt to respond to a need of an increasing number of laboratories stopping TSE activity.
Biological Risk Assessment
Pathogenesis
TSEs are a group of transmissible neurodegenerative diseases in mammals characterized by spongy degeneration of the brain with severe neurological symptoms (). Until now, the issue of disease is always fatal. Strong evidence indicated that prion is the causative agent of TSE and seems to act in the same way in all these diseases. In an attempt to explain the molecular basis of TSE-associated neurotoxicity, Alper and Griffith proposed in the 1960s the implication of an infectious agent made solely of protein.Citation11,Citation12 In the 1980s Prusiner and his team isolated the infectious protein particle he named “prion” for “proteinaceous infectious particle.”Citation13 The prion protein-only hypothesis proposed that normal cellular prion protein (PrPc) acts as a template for post-translational conversion of PrPc into prion (PrPsc), an abnormal misfolded isoform of PrPc causing scrapie in sheep. The new generated PrPsc has different biochemical characteristics compared with PrPc, such as its insolubility, resistance to denaturation and its partial resistance to protease degradation. PrPsc was predicted to propagate during infection by contacting specific regions on PrPc to recruit this protein and convert it in PrPsc. Polymers so formed undergo a fragmentation resulting in generation of new templates or new “seeds” for prion fibril formation.Citation14
PrPc is normally highly expressed within the nervous system with variation among distinct brain regions and different cell types. Various cellular components of the immune system, in the bone marrow, blood and peripheral tissues also express substantial amounts of PrPc.Citation15 Most mammalian PrPc is exported to the cell surface as a glycoprotein with GPI anchor domain in phospholipids bilayer and might have a pleiotropic role in vivo perhaps by mediating its broad effects by affecting cell signaling pathways.Citation16 It has been shown to participate in normal cellular functions including cell signaling, metal homeostasis, protection against apoptosis and oxidative stress, neurite growth, neurogenesis and neuroprotection.Citation15,Citation17 The pathogenesis of prion diseases is attributed to major changes in the metabolism of PrPc with a functional role for prion in TSE etiology.Citation16
Recent data suggest a tight and specific interaction between Amyloid-β oligomers (Aβ) and PrPc (and not PrPsc) in mediating Alzheimer disease.Citation18,Citation19 However some conflicting reports exist where the effects of Aβ in memory properties have been shown to be independent of PrPc.Citation20,Citation21 Thus more research is needed to establish the link between PrPc and Alzheimer disease.
Prion propagation in brain proceeds via 2 distinct phases during the disease: a clinically silent exponential phase not rate-limited by prion protein concentration, which rapidly reaches a maximal prion titer, followed by a switch to a plateau phase. It is suggested that prions themselves are not neurotoxic but catalyze the formation of such toxic species from PrPc. This production is triggered when prion propagation saturates and leads to a switch from autocatalytic production of infectivity (phase 1) to a toxic pathway (phase 2).Citation22
The particular nature and origin of prion impose researchers to come out the ordinary precepts of an infection and to understand new concepts involving a cellular protein belonging to the infected organism itself. Prion protein carries characteristics of neurodegenerative diseases such as Alzheimer and Parkinson Diseases where abnormal misfolding and aggregation of native proteins occur leading to damage in brain tissues and disturbances in the normal cellular protein function.Citation23 Prion also possesses characteristics of an infectious microorganism with the capacity to transmit to other organisms of the same species or to a different species.
Infectious dose
Regarding the infectious dose, it is admitted that prions are infectious at very low concentrations. By comparing the scrapie dose-response curve observed in mice to model predictions, Fryer and McLean found no evidence of the existence of a threshold for infectiousness, the probability of infection simply becomes smaller as the dose decreases.Citation24 This is supported by a study in hamsters administrated orally with a scrapie infected brain homogenate; in this model lethal dose was found to be close to the infectious dose.Citation25
The prion median lethal dose (LD50) may depend on the experimental animal model and the prion strain used in studies. The inoculation route of transmission (orally or by injection) as well as the inoculation site (intraperitoneal or intracerebral) are factors influencing also the LD50.Citation26
Interestingly, researchers have observed a high scrapie incidence in mice receiving repeated intraperitoneal injections of very low scrapie prion doses.Citation26 The more frequent the inoculations, the higher the scrapie incidence. The observation was also reported in oral scrapie infection experiments of hamsters.Citation27 The same total doses inoculated in a single challenge do not induce the disease suggesting that a degradation mechanism of PrPsc is able to clear a single low prion dose but saturates when animals are subjected to repeated exposures.Citation26
Therapeutics and vaccines
Until today no therapeutic treatments or prophylaxis have been proved to be efficient enough to treat TSEs. However investigations in mice suggest that different therapeutic ways may prevent or delay the onset of prion diseases.
Chemotherapies have focused on blocking the conversion of the normal form of prion protein to its abnormal PrPsc form.Citation28 This is achieved either by directly binding PrPc or PrPsc, or by redistributing, sequestering, or downregulating PrPc. Other strategies aim to enhance the clearance of PrPsc or to influence cell-signaling molecules, which are required for pathogenesis.Citation29
Other promising therapeutic and prophylaxis approaches aimed to block PrPsc are based on PrP RNA interference, passive or active immunization, dominant negative inhibition of PrPsc formation or aimed to inhibit interactions between PrPsc and other cofactors.Citation29-Citation31 Recently a study showed that intracerebral transplantation of fetal neural stem cells significantly extended the survival time in mice and may represent an efficient alternative therapy against prion diseases.Citation32
Transmission
TSE can occur as a result of an as yet uncharacterized sporadic event causing PrPc to PrPsc conversion, or by dominant mutations in the PRNP gene encoding PrP, producing mutant PrPc that is hypothesized to more readily undergo spontaneous conversion to PrPsc. The PRNP gene is highly variable in humans and in various animal species. Numerous mutations and polymorphic sites have been described. Data demonstrate the influence of PRNP variations in conditioning the susceptibility to and the clinical and pathological phenotype of prion diseases, their pathogenesis as well as the selection and mutation of prion strains.Citation33 It is suggested that sequence variants exert their effects by altering the efficiency of conformational self-replication and they do so by targeting different steps in this process.Citation16 In humans, the susceptibility to prion disease is considerably increased by the valine/methionine polymorphism in position 129 of the prion protein.Citation34 The emergence of new strains is often related with PRNP variation, which can drive the evolution of strains both on interspecies transmission and on transmission within species.Citation18,Citation35
TSE is also the unique neurodegenerative disorder that can be caused through experimental and natural infection with exogenous prions either by feeding as in the case of vCJD and Kuru, or by deep body contact with prion infected material such as in surgery or invasive treatments.Citation36
In humans, iatrogenic CJD (iCJD) transmission cases were found to occur through different ways: by parenteral administration of cadaveric-derived growth hormone, by blood transfusion, through intracerebral dura mater grafting or by the use of neurosurgical instruments and EEG electrodes.Citation37 Transmission through corneal transplantation and during endodontic treatment was also described.Citation38 These observations correlate with the wide variety of tissues in variant CJD (vCJD) showing infectivity and presence of PrPsc: the central nervous system (CNS), the LRS system (spleen, lymph nodes, tonsil, appendix, other gut-associated lymphoid tissues), blood, components of the eye and optic nerve, and the gastrointestinal tract. This is in contrast with sporadic CJD (sCJD) or inherited prion diseases in which the infectious material is largely confined to the tissues of the CNS.Citation36
In animals, TSEs (TME, CWD, BSE, FSE, and scrapie) are thought to occur naturally after consumption of prion-infected foodstuffs. However, experimental transmission has been routinely achieved via intraperitoneal and intravenous injection of prion infected material. Transmission was reported to be effective by intralingual, intranerval, conjuntival, and nasal cavity inoculations.Citation39 Prion tissue distribution in cattle remains essentially restricted to the CNS, cattle have no evidence of a lymphoid or blood phase of PrPsc. This is fundamentally different from TSEs in sheep, cervids or mice, and vCJD in human. Sheep and cervids appear to have extensive lymphoid tissue involvement with PrPsc deposition regardless of the TSE agent they are inoculated with.Citation17
Prion infectivity has been detected in some body fluids in animals: cerebrospinal fluid, blood, saliva, milk and urine raising the possibility of prion shedding in these liquid secretions and excretions. All fluids can act as sources of aerosols and may represent a point of origin for airborne transmission of disease. Recently, exploring the aerosol transmission potential of prions, a study showed that mouse scrapie could efficiently transmit via aerosols to mice.Citation40 In deer some observations favor the airborne transmission of chronic wasting disease (CWD) as naturally occurring and has been shown to occur experimentally on “cervidized” transgenic mice.Citation41 Saliva and droppings were found to harbor CWD infectivity. In addition, CWD prion has been found in water in the natural environment in an endemic area.Citation39,Citation42 Infectivity has not yet been demonstrated in milk and blood of cattle with natural or experimental BSE.Citation17 In the case of CJD, there is no evidence of release of prion into aerosols.
Although the BSE epidemic and the transmission of the agent to human demonstrate that prions can pass the species barrier, the cross-species transmission is much less efficient than within the same species and strain characteristics may change on transmission to another species.Citation43 The species barrier that limits the cross-species transmission of prions may be due to differences in the amino acid sequences of PrP with certain residues position having a strong influence and is thought to depend on the conformational compatibility between the exposed host PrPc and the infecting PrPsc.Citation44 The “conformational-selection” model proposes that prions may be endowed with a variety of PrPsc conformers, the fittest conformation being selected in a particular environment or tissue.Citation18,Citation45 The European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC) published in 2011 a scientific opinion on possible epidemiological and molecular association between TSEs in animals and human, making a state-of-the-art on current knowledge on risks of interspecies transmission.Citation2 Based on the results from in vitro experiments and transmission experiments to human PrP transgenic mice, the report states that the human prion protein can be converted to a PrPsc-like form by animal PrPsc: there is not an absolute barrier to infection/conversion of humans by mammalian TSEs at the molecular level. Based on epidemiologic observations, BSE is considered the most promiscuous TSE strain transmitting to humans, cats and zoo animals.Citation44
Recently Béringue and colleagues showed that prion cross-species transmission efficacy could exhibit marked tissue dependence.Citation46 They observed a higher permissiveness of the spleen over the brain tissue to foreign prions and PrPsc amplified in spleen was able to re-infect the donor species efficiently. These data could explain discrepancy observed between numbers of clinical vCJD cases and estimates of population prevalence of infection in LRS tissues: BSE exposure led to LRS colonization but progress to neuroinvasive disease is rather low.Citation43
Persistence in the environment
BSE and vCJD are two epidemics that seem to have been sustained by prion contamination in feed and food chains and no horizontal transmission has been described. Conversely, animal to animal transmission through the environment contributes to maintain epizootics of scrapie in sheep and CWD in deer and elk populations. Prions may be deposited in the environment through the remains of dead animals and via urine, saliva, and other body fluids. They are then maintained in soil by binding to mineral particles and other soil components. These associations have been shown to enhance the persistence and surprisingly the transmissibility of the infectious agent.Citation47-Citation49 Studies have shown that in sewage and seawater the survival of PrPsc is limited to 2 to 4 months. In sewage, the stability of PrPsc associated to BSE is significantly lower than that reported for PrPsc associated to sheep and mouse scrapie.Citation50
Prion risk classification
Prions are categorized as unconventional agents associated with TSE as they are infectious particles derived from an organism protein devoid of nucleic acids. TSE causing agents are classified in class of risk 3 for humans and animals by most of international pathogenic organism classifications (Directive EC 2000/54; Approved List of Biological Agents, UK; List of viruses and unconventional agents, Belgium).Citation9,Citation51,Citation52 Switzerland makes exception with scrapie and CWD classified in class of risk 2 for humans.
Despite large gaps in prion disease knowledge, relevant characteristics have been delimited during last years that confirm the risk class of TSE causing agents. First, it was already known that irrespective of the infected species prions cause irremediably death and today no treatment is available to cure or prevent the disease development.
Much progress has been made in the understanding of transmission of prion, a relevant characteristic for its classification that takes into account the (pathogen) route of transmission, its infectious dose, and the capacity to persist infectious outside a host. As mentioned above, natural transmission seems to take place mainly via oral route in human and animals. Besides this principal route of transmission prion potential to transmit experimentally has been shown to occur by different routes. In particular scrapie aerosol transmission has been achieved experimentally in mice.Citation39,Citation40 In cervids, CWD was already proposed as a natural airborne pathogen.Citation41 The prion low infectious dose and the high persistence into the environment increase the exposure to new hosts. As prion persistence and infectivity seem to be increased when attached to soil components exposure may even be increased in animals.Citation48 Data reported in human suggest the possibility of a silent infection rate in the community exposed to BSE dietary.Citation46,Citation53,Citation54 As iatrogenic transmission has been shown to occur by different routes and human prion has been shown to be largely distributed in tissues in humans with vCJD, this situation represents a particular issue in medical and dentistry sectors.
Zoopathogen classification takes also into account the economic loss factor and the consequences of diseased animals for livestock farms. It has to be mentioned here that the BSE epidemic is today at its lowest number of cases in Europe since the beginning of the mad cow disease crisis.
Finally prion is prone to “mutation” and can adapt to new host environment. Until now how this adaptability proceeds is unknown. These prion characteristics require to maintain high attention and epidemiologic surveillance in human and animals.
Diagnostic and research activities related to TSE causing agents
TSE diagnostic activity in Europe has been incorporated within the framework of BSE and vCJD surveillance programs. Since the principal transmission barrier of concern to public health is the one between humans and cattle BSE prions, an important part of diagnostic activity is focused on rapid detection of BSE in human foodstuff. Scrapie diagnostic is performed because the disease constitutes a substantial economic loss to producers. Historical TSE disease confirmation was based on the demonstration of the morphological features of spongiform encephalopathy by histopathological examination of the brain. As PrPsc is widely accepted as a consistent disease marker, all current diagnostic methods of TSE are based on the demonstration of the presence of PrPsc: Enzyme-linked immunosorbent assay (ELISA), lateral flow device (LFD), rapid western blot are commonly used as rapid tools to screen samples. They are used as part of confirmatory process.Citation55 No diagnostic test is currently available for live animals and post-mortem brain samples are still necessary. Studies have however reported development of techniques based on the histological detection of prion in lymphoid tissues with PrP-specific antibodies allowing for vCJD to be diagnosed reliably simply on a tonsil biopsy.Citation56 However, these methods are still time consuming.
Much progress has been made to develop new techniques aiming to reduce detection limits, assay cost and time. Transmission from infected brain tissue in animal bioassays is the main method currently available for detection of infectivity and for characterization of strains. However, this method is slow, rather imprecise, requires large number of animals as well as dedicated animal facilities increasing the risk for workers. Alternatives have been found such as the cell-based titration procedures in which high-sensitive cell lines are inoculated with a prion strain and after an incubation period, cells are directly tested for prion quantitative detection.Citation57,Citation58 Protein misfolding cyclic amplification (PMCA) relies on the structural transition of PrPc to PrPsc catalyzed by small amounts of PrPsc present in brain homogenate and proceeds in cycles of incubation and sonication.Citation59 PMCA has the lowest reported PrPsc detection limit. Real-time quaking-induced conversion (RT-QuIC) and enhanced QuIC are based on same principle as PMCA but here recombinant prion protein (recPrP) replaces the source of naturally occurring PrPc and controlled periodic shaking replaces sonication. These techniques allow relative sensitive quantitative detection of prion seeding activity in various samples including blood plasma, cerebral spinal fluid and nasal fluids.Citation60 They are currently used in research studies on prion nature and replication, significance of strain differences, to understand transmission and species barrier as well as the influence of PRNP polymorphism. They require validation to be used in animal or human TSE diagnostic in the framework of TSE surveillance programs. Indeed in Europe TSE diagnostic of animals and humans follow precise standard procedures and take place within a quality system. Procedures are well established and validated.
Risk management of contained use activities involving TSE causing agents
Human and animal research and diagnostic activities should handle prion material in a contained facility that guarantees protection of human health and the environment. The containment level to adopt takes into account the prion nature, infectivity characteristics, the routes of exposure for workers and its well-known resistance to classic decontamination methods. Protective measures must also be adapted to the type of infectious material handled, the nature of the manipulation and the amount of material handled. The Biosafety in Microbiological and Biomedical Laboratories manual recommends that manipulation of prions in research and diagnostic laboratories takes place in a containment level 2 or 3 depending on the type of activity.Citation61 Most activities involving prions are however conducted in containment level 3 facilities.
As mentioned earlier, the European Directive 2000/54/CE classifies TSE causing agents in class of risk 3. The Directive distinguishes these agents by two asterisks (**) meaning they may present a limited risk of infection for workers because they are not normally infectious by the airborne route. In these conditions and after risk assessment of the specific activity Member States may be dispensed to apply certain containment measures. In Belgium, diagnostic activities handling brain material in the frame of the epidemiology surveillance program of TSE in animals must be performed in a containment level 3 facility adapted to BSE work (also noted L3-BSE, see for details). In the L3-BSE laboratory negative pressure differential between the room and the adjacent area, HEPA filtration of exhaust air and airtightness of the room are not required. Moreover the resistance of the prion protein to formaldehyde exclude the use of it for fumigation. Today, alternatives such as hydrogen peroxide are proposed, but its use still needs validation for decontamination of facilities handling prions.
Table 3. Design features, technical characteristics, safety equipment, work practices and waste (disposal) management required in Belgian laboratories that perform rapid BSE detection testing
Recent data on animal experiments suggest potential contamination with prions through the inhalation route and may question L3-BSE containment for diagnostic activities involving animals (bioassays).Citation39-Citation41 More studies are needed to understand how prion can infect a host by inhalation in laboratory conditions in order to define if a proper containment level 3 will also be required for diagnostic activities. Nevertheless, in diagnostic laboratories, sample preparation involving grinding and homogenization of potentially contaminated brain tissue generates infectious droplets and aerosols and should be performed in closed systems including the use of a biosafety cabinet and appropriate personal protection (respiratory mask, eye protection, see annex 1). The risks linked to further treatment of homogenate to detect PrPsc are accidental parenteral inoculation or ingestion and should also be considered and prevented. Adoption of Containment level 3 work practices and waste management and a stringent quality assurance system imposing standardization of protocols will respectively ensure biosafety and liability of results. Lastly implementation of a global biorisk management system in the organization such as the CEN Workshop Agreement 15793:2011 integrating biosafety and biosecurity management may reinforce protection of workers, public health and of the environment.Citation62
Compared with diagnostic activities, research activities represent a bigger challenge as research methods are not standardized and may involve the use of experimental animals increasing the risk of exposure. To deal with these uncertainties research is conducted in a proper containment level 3 laboratory and animal house.
Inactivation of TSE causing agents
In a laboratory handling TSE causing agents protection of public health and environment requires a special attention to decontamination procedures and waste management. Indeed, resistance of prions to classic treatments is well-known and therefore resistant misfolded prion is noted PrPres in most of inactivation studies. Lots of research efforts have been dedicated to develop efficient decontamination methods as an absolute decontamination is necessary because of the low infectious dose of TSE causing agents. As a matter of fact incineration remains the safest elimination method of prion-contaminated material and biological waste. Alternatively and particularly for big animal carcasses, alkaline hydrolysis in high temperature and pressure conditions (tissue digester) may be proposed as a proven effective inactivation method.Citation63,Citation64
PrPres is a hydrophobic protein that tends to self-aggregate giving high stability to the protein. The misfolded prion form has a significantly higher ratio of β-sheets and lower ratio of α-helical structures than the cellular form. The conformational change from helices to β-sheets confers particular physico-chemical properties to the prion protein which becomes insoluble and resistant to classic treatments (with protease, heat, or radiation) although they reduce prion infectivity.Citation65 Furthermore TSE agents have been shown experimentally and clinically to gain resistance to decontamination treatments and to efficiently transfer infectivity to a suitable host when adsorbed on metal or soil components.Citation66,Citation67
The clinically silent infection with vCJD prions in populations exposed to BSE prions as well as wide tissue distribution of infectivity in vCJD make the risk of infection through contact with medical instruments a major concern. That’s the reason to the large number of research and development studies being focused on medical instrument decontamination. Contained use activities handling prions benefit from these data as they also require decontamination methods to apply to any material that must be re-used, surfaces that must be decontaminated and to waste inactivation. In 2000, the WHO published infection control guidelines for known and suspected TSE cases.Citation68 These guidelines propose safety measures to be applied in case of human TSE agent’s manipulation including criteria for identification of risk groups, special consideration of surgical procedures associated with high-risk tissues and specialized cleaning and decontamination practices. The decontamination method recommended by WHO for prion consists on a sequential treatment of immersion for one hour in high concentration of sodium hypochlorite (NaOCl) solution or sodium hydroxide (NaOH) solution followed by autoclaving at a temperature of 121 °C for 30 minutes.Citation68 Because of the corrosive effect of these concentrations of NaOH and NaOCl on metal instruments as well as their harmful consequences on worker health and the incompatibility of autoclaving with delicate surgery instruments research has been focused on finding alternatives for surgical instruments.
Taylor and more recently Rutala and Weber have reviewed physical and chemical methods of prion decontamination described in the literature and the resulting infectivity status ( and ).Citation65,Citation69 They describe relevant factors affecting prion protein inactivation including prion strain (different thermostability or resistance to proteases), the nature of the surface concerned (plastic or stainless steel), and the method of sample preparation (undiluted tissue, tissue homogenate, tissue macerate). The fixation and hydration states of the agent and the size and nature of the inoculum, in particular the lipid content, have been shown to influence efficiency of prion inactivation by heat and reactive oxygen species.Citation70,Citation71
Table 4. Efficacy of sterilization processes in inactivating prions
Table 5. Efficacy of chemicals in inactivating prions
Studies have shown the effectiveness of specific formulations of alkaline and enzymatic detergents to eliminate the infectivity of prions with the advantage of being applicable to delicate surgical instruments and medical devices and far less hazardous to operators.Citation69,Citation72 Detergent enhances action of sodium hydroxide by liberating lipid membrane-protected PrPres and thus improves access to NaOH which can then inactivate PrPres by altering its structure.Citation73 Also the use of specific formulations of alkaline or enzymatic detergents in combination with standard sterilization processes have already led to the development of validated methods for the sterilization of prion-contaminated medical devices.Citation69 Using Standard Steel-Binding Assay (SSBA), a highly sensitive detection method, commercially available decontamination reagents have been compared.Citation36 The most effective reagents obtain a residual infectivity below the limit of detection (a reduction of prion infectivity of 8 logs).
Relevant papers on prion inactivation using hydrogen peroxide (H2O2) vapor or reactive oxygen species (ROS) generated by various systems suggest that ROS can be a main actor in prion inactivation. Sterilization methods using oxidizing agents include ozone (TSO3), a mix of hydrogen peroxide and peracetic acid, and hydrogen peroxide with copper, the hydrogen peroxide gas plasma system in particular conditions of humidity, temperature, pH and contact time.Citation67,Citation71,Citation74,Citation75 Several studies have confirmed that new low-temperature sterilization technologies (ie, gas plasma and vaporized hydrogen peroxide) can eliminate infectivity of prions on stainless steel wires and may be useful for reducing or preventing risk associated with prion-contaminated devices. These technologies, combined with use of prionicidal detergents, should sterilize prion-contaminated heat-sensitive devices, but use of these technologies should wait for corroborative studies to be published.Citation66,Citation69,Citation76
Decommissioning of a TSE diagnostic laboratory
As a consequence of a decreasing number of detected cases of TSE infection in cattle in the recent years, the European Commission authorized some Member States to reduce their diagnostic activities in the framework of the European Surveillance Program for TSEs. This is the case of Belgium. Consequently the Belgian competent authority, the Federal Agency for the Safety of the Food Chain (FAFSC), decided to decrease the number of national laboratories performing rapid detection of TSEs in animal tissues from Bovidae, Ovidae, and Caprinae. However the question was raised about the best procedure to apply for decommission of these laboratories.
A step-by-step risk assessment performed before lab dismantlement could lead to a procedure specific for the concerned laboratory.Citation77 This assessment would take into account the present and future activities to be performed in the laboratory. When a high number of positive specimens as well as high concentrations of infectious material have been handled in the lab during the time of prion detection activity, the probability of room exposure to prion might be high. The number and type of spills or other incident that occurred in the laboratory are additional factors increasing the probability of room or equipment contamination. In that case a complete destruction of all material, equipment and furniture might be considered. On the other hand for a diagnostic laboratory in which a low number of positive samples have been handled, with no incident registered during the time of prion detection activity and with a rigorous quality system or (and) a biorisk management system in place, decommissioning could be limited to decontamination of surfaces and disposal of devices that have been directly in contact with potentially infectious material. But laboratories which did not have any positive case have to be aware of the fact that they participated in the yearly national ringtrials organized by the National Reference Laboratory in which positive material of actual cases was always included.
The future activity planned for the laboratory may also influence the decommissioning procedure. In case the laboratory will be used for administrative work for example complete destruction should also be advised. In case the future activity will require a containment level 3, the work environment and particularly the work practices will remain stringent and will reduce the possibility of exposure to remaining contaminating prions. However standard decontamination methods usually applied in containment level 3 laboratories for conventional microorganisms do not ensure effective decontamination of prions and may even reinforce the resistance of prions to disinfectants.
Nevertheless in most of the cases, the precautionary principle will lead to a procedure prescribing complete destruction of all material, equipment and furniture. Aside from the fatal issue of prion exposure (particularly BSE) and remaining questions about the biology of TSE causing agents, there are practical considerations that favor this decision. The probability of exposure to prion protein is high as it can remain infectious in the lab environment for a long time when attached to soil, metals, and other components.Citation67,Citation78 It is known that transmission of disease is achieved with tiny amounts of infected tissue so complete decontamination is required. The standard methods for decontamination are not efficient against the prion protein. Absence of sampling and detection methods specific and sensitive enough to detect traces of the prion protein in the work environment and providing a proof of decontamination complicates the decommissioning task. Progress has been recently achieved in this field. A research team provided a method with a high dynamic range and a sensitivity beyond that of conventional rodent bioassay.Citation36 Finally a recent study supports the proposal of complete destruction. It has compared different pen treatments in a farm with high incidence of natural scrapie: pressure washing alone, pressure washing combined with sodium hypochlorite treatment, the same plus replacement of all removable metalwork, re-galvanizing of remaining metalwork, and a complete repaint of whole pen including the walls and floor. None of these treatments were efficient enough for environmental decontamination.Citation79
The FAFSC, the Belgian National Reference Laboratory for TSE and the Biotechnology and Biosafety Unit (SBB) of the Scientific Institute of Public Health cooperated to establish a procedure for dismantlement based on the present knowledge on TSE (). A technical document proposes a dismantlement procedure of laboratories performing rapid diagnosis of TSE and the safety measures to adopt during the process.Citation80 The procedure of dismantlement includes the following steps (in this order): (1) A cleaning step to eliminate any residual organic matter that can interfere with the disinfectant action; (2) Decontamination of prions using high concentrations of disinfectant solutions such as sodium hydroxide (NaOH) and sodium hypochlorite (NaOCl); (3) Packing of decontaminated equipment and furniture in compliance with international transport regulations prior to incineration by a waste processing company which disposes of an incinerator for hazardous waste; (4) Room fumigation with hydrogen peroxide.
Figure 1. Procedure for decontamination and dismantlement of laboratories performing rapid detection of Transmissible Spongiform Encephalopathy. Dismantlement starts with equipment and furniture in the room and finishes with decontamination of the emptied room. Small equipment is disposed in a biohazard container without prior decontamination and transported to the incinerator. Large equipment, walls, floor and fixed furniture are cleaned with a detergent and then vaporized (humidified) with NaOH 2N or NaOCl 20 000 ppm for one hour. Surfaces are then rinsed with water. Large equipment is packed in 2 layers of resistant plastic film, placed in cardboard packaging and transported to the incinerator. The room is made airtight and fumigated with hydrogen peroxide. In case of “small” incinerator oven entrance, two solutions are proposed: large equipment is cut in the laboratory into pieces small enough to enter the oven, or large equipment is crushed just before entering the oven at the incineration site. Small and large equipment are incinerated in a specialized incinerator for hazardous waste.
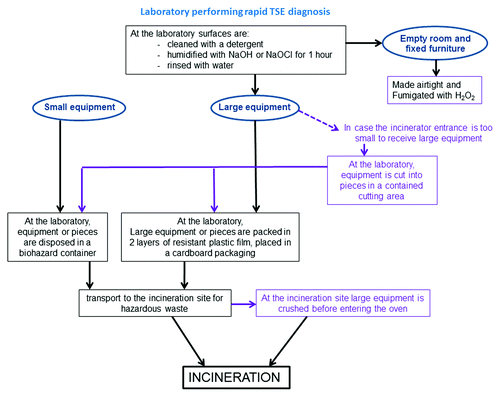
Particular attention is paid on safety measures to apply during the decommissioning of the laboratory. The procedure should be adapted later on taking into account the evolution of scientific knowledge on the subject.
Conclusion
This paper gives a review of the current knowledge on prionology, TSE pathogenesis and discuss the state-of–the-art of risk evaluation and risk management of research and diagnostic activities. Important questions are raised with respect to biosafety: is prion airborne and transmissible by inhalation of infectious aerosols? How does a prion strain cross the species barrier? How to completely eliminate prion contamination from instruments, devices, other surfaces? Special attention was paid to new knowledge on inactivation of prion, a crucial step for protection of human health and environment in contained use activities such as animal and human diagnostic and research. More particularly, this becomes an important issue in the frame of decommissioning of laboratories closing down their diagnostic activities as a result of a decreasing number of detected cases of TSE infection.
Further investigation on these questions might lead to re-assessment of the risks linked to manipulation of prions and to an adaptation of the containment measures in place today.
| Abbreviations: | ||
| Aβ | = | Amyloid-β oligomers |
| BSE | = | bovine spongiform encephalopathy |
| CJD | = | Creutzfeldt-Jacob disease |
| CNS | = | central nervous system |
| CWD | = | chronic wasting disease |
| FSE | = | feline spongiform encephalopathy |
| GPI | = | glycophosphatidylinositol |
| LRS | = | lymphoreticular system |
| Prion | = | proteinaceous infectious particle |
| PrP | = | prion protein |
| PrPc | = | cellular prion protein, non-pathogenic form of prion |
| PrPres | = | prion protein resistant to proteinase K treatment |
| PrPsc | = | prion protein responsible of scrapie in sheep |
| PrPcwd | = | prion protein responsible of chronic wasting disease in cervids |
| sCJD | = | sporadic Creutzfeldt-Jacob disease |
| vCJD | = | variant Creutzfeldt-Jacob disease |
| TME | = | transmissible mink encephalopathy |
| TSE | = | transmissible spongiform encephalopathy |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Katia Pauwels, Didier Breyer and Martine Goossens (Scientific Institute of Public Health, Brussels, Belgium) and Willy Zorzi (University of Liège, Liège, Belgium) for their useful contribution to this document. This work received support from the Brussels-Capital Region (IBGE-BIM), the Flemish Region (LNE) and Wallonia (DGARNE).
References
- Bradley R, Collee JG, Liberski PP. Variant CJD (vCJD) and bovine spongiform encephalopathy (BSE): 10 and 20 years on: part 1. Folia Neuropathol 2006; 44:93 - 101; PMID: 16823691
- EFSA panel on biological hazards and ECDC. Joint scientific opinion on any possible epidemiological or molecular association between TSEs in animals and humans. EFSA Journal 2011; 9:1945
- Collee JG, Bradley R, Liberski PP. Variant CJD (vCJD) and bovine spongiform encephalopathy (BSE): 10 and 20 years on: part 2. Folia Neuropathol 2006; 44:102 - 10; PMID: 16823692
- Prusiner SB. Prion diseases and the BSE crisis. Science 1997; 278:245 - 51; http://dx.doi.org/10.1126/science.278.5336.245; PMID: 9323196
- European Commission. Regulation n°999/2001 of the European Parliament and the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Official Journal of the European Communities 2001;L147.
- Smith PG. The epidemics of bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease: current status and future prospects. Bull World Health Organ 2003; 81:123 - 30; PMID: 12751420
- European Commission, Directorate-General for Health & consumers. The TSE Roadmap 2. 2010 Jul 16. Report: COM 2010;384.
- EFSA. Scientific and technical assistance on the minimum sample size to test should an annual BSE statistical testing regime be authorized in healthy slaughtered cattle. EFSA Journal 2012; 10:2913
- European Commission. DIRECTIVE 2000/54/EC of 18 September2000 on the protection of workers from risks related to exposure to biological agents at work. Official Journal of the European Communities 2000;L262.
- European Commission. Directive 2009/41/EC of the European Parliament and of the Council of 6 May 2009 on the contained use of genetically modified micro-organisms. Official Journal of the European Union 2009; L125:75
- Alper T, Cramp WA, Haig DA, Clarke MC. Does the agent of scrapie replicate without nucleic acid?. Nature 1967; 214:764 - 6; http://dx.doi.org/10.1038/214764a0; PMID: 4963878
- Griffith JS. Self-replication and scrapie. Nature 1967; 215:1043 - 4; http://dx.doi.org/10.1038/2151043a0; PMID: 4964084
- McKinley MP, Masiarz FR, Prusiner SB. Reversible chemical modification of the scrapie agent. Science 1981; 214:1259 - 61; http://dx.doi.org/10.1126/science.6795721; PMID: 6795721
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science 2007; 318:930 - 6; http://dx.doi.org/10.1126/science.1138718; PMID: 17991853
- Linden R, Martins VR, Prado MAM, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev 2008; 88:673 - 728; http://dx.doi.org/10.1152/physrev.00007.2007; PMID: 18391177
- Tuite MF, Serio TR. The prion hypothesis: from biological anomaly to basic regulatory mechanism. Nat Rev Mol Cell Biol 2010; 11:823 - 33; http://dx.doi.org/10.1038/nrm3007; PMID: 21081963
- Hamir AN, Kehrli ME Jr., Kunkle RA, Greenlee JJ, Nicholson EM, Richt JA, Miller JM, Cutlip RC. Experimental interspecies transmission studies of the transmissible spongiform encephalopathies to cattle: comparison to bovine spongiform encephalopathy in cattle. J Vet Diagn Invest 2011; 23:407 - 20; http://dx.doi.org/10.1177/1040638711403404; PMID: 21908269
- Radford H, Moreno J, Peretti D, Verity N, Guerra Martin MSJ, Mallucci G, et al. The role of PrP in synaptic function and repair - Implications for treatment of prion and Alzheimer diseases. Prion 2012 Conference 2012;6:2-22.
- Tatzelt J. The cellular prion protein mediates neurotoxic signaling of scrapie prions and Amyloid-beta. Prion 2012 Conference 2012;6:2-22.
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, et al. Synthetic amyloid-β oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A 2010; 107:2295 - 300; http://dx.doi.org/10.1073/pnas.0911829107; PMID: 20133875
- Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature 2010; 466:E3 - 4, discussion E4-5; http://dx.doi.org/10.1038/nature09217; PMID: 20703260
- Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature 2011; 470:540 - 2; http://dx.doi.org/10.1038/nature09768; PMID: 21350487
- Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 2004; 6:1054 - 61; http://dx.doi.org/10.1038/ncb1104-1054; PMID: 15516999
- Fryer HR, McLean AR. There is no safe dose of prions. PLoS One 2011; 6:e23664; http://dx.doi.org/10.1371/journal.pone.0023664; PMID: 21858197
- Baier M, Norley S, Schultz J, Burwinkel M, Schwarz A, Riemer C. Prion diseases: infectious and lethal doses following oral challenge. J Gen Virol 2003; 84:1927 - 9; http://dx.doi.org/10.1099/vir.0.19037-0; PMID: 12810889
- Jacquemot C, Cuche C, Dormont D, Lazarini F. High incidence of scrapie induced by repeated injections of subinfectious prion doses. J Virol 2005; 79:8904 - 8; http://dx.doi.org/10.1128/JVI.79.14.8904-8908.2005; PMID: 15994784
- Diringer H, Roehmel J, Beekes M. Effect of repeated oral infection of hamsters with scrapie. J Gen Virol 1998; 79:609 - 12; PMID: 9519841
- Sim VL, Caughey B. Recent advances in prion chemotherapeutics. Infect Disord Drug Targets 2009; 9:81 - 91; http://dx.doi.org/10.2174/1871526510909010081; PMID: 19200018
- Ramachandran TS. Prion-related diseases. [Cited 2012 May 2]; Available from:.
- Buchholz CJ, Bach P, Nikles D, Kalinke U. Prion protein-specific antibodies for therapeutic intervention of transmissible spongiform encephalopathies. Expert Opin Biol Ther 2006; 6:293 - 300; http://dx.doi.org/10.1517/14712598.6.3.293; PMID: 16503737
- Kalinke U, Bach P, Koning M, Buchholz CJ. Vaccination against prion diseases. [Cited 2009 July 27]; Available from:
- Relaño-Ginés A, Lehmann S, Bencsik A, Herva ME, Torres JM, Crozet CA. Stem cell therapy extends incubation and survival time in prion-infected mice in a time window-dependant manner. J Infect Dis 2011; 204:1038 - 45; http://dx.doi.org/10.1093/infdis/jir484; PMID: 21881119
- Morales R, Abid K, Soto C. The prion strain phenomenon: Molecular basis and unprecedented features. Biochimica et Biophysica Acta (BBA) -. Molecular Basis of Disease 2007; 1772:681 - 91; http://dx.doi.org/10.1016/j.bbadis.2006.12.006
- Owen F, Poulter MCJ, Collinge J, Crow TJ. Codon 129 changes in the prion protein gene in Caucasians. Am J Hum Genet 1990; 46:1215 - 6; PMID: 2378641
- Agrimi U. PrnP and susceptibility to prion diseases - from resistance to spontaneous disease. Prion 2012; 6:2 - 22
- Edgeworth JA, Sicilia A, Linehan J, Brandner S, Jackson GS, Collinge J. A standardized comparison of commercially available prion decontamination reagents using the Standard Steel-Binding Assay. J Gen Virol 2011; 92:718 - 26; http://dx.doi.org/10.1099/vir.0.027201-0; PMID: 21084494
- Head MW, Ironside JW. Review: Creutzfeldt-Jakob disease: prion protein type, disease phenotype and agent strain. Neuropathol Appl Neurobiol 2012; 38:296 - 310; http://dx.doi.org/10.1111/j.1365-2990.2012.01265.x; PMID: 22394291
- Bourvis N, Boelle PY, Cesbron JY, Valleron AJ. Risk assessment of transmission of sporadic Creutzfeldt-Jakob disease in endodontic practice in absence of adequate prion inactivation. PLoS One 2007; 2:e1330; http://dx.doi.org/10.1371/journal.pone.0001330; PMID: 18159228
- Stitz L, Aguzzi A. Aerosols: an underestimated vehicle for transmission of prion diseases?. Prion 2011; 5:138 - 41; http://dx.doi.org/10.4161/pri.5.3.16851; PMID: 21778819
- Haybaeck J, Heikenwalder M, Klevenz B, Schwarz P, Margalith I, Bridel C, Mertz K, Zirdum E, Petsch B, Fuchs TJ, et al. Aerosols transmit prions to immunocompetent and immunodeficient mice. PLoS Pathog 2011; 7:e1001257; http://dx.doi.org/10.1371/journal.ppat.1001257; PMID: 21249178
- Denkers ND, Seelig DM, Telling GC, Hoover EA. Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J Gen Virol 2010; 91:1651 - 8; http://dx.doi.org/10.1099/vir.0.017335-0; PMID: 20164261
- Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, Gertig K, Hoover EA, Jewell JE, Telling GC, Zabel MD. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 2009; 3:171 - 83; http://dx.doi.org/10.4161/pri.3.3.9819; PMID: 19823039
- Collinge J. Cell biology. The risk of prion zoonoses. Science 2012; 335:411 - 3; http://dx.doi.org/10.1126/science.1218167; PMID: 22282797
- Bett C, Fernández-Borges N, Kurt TD, Lucero M, Nilsson KP, Castilla J, Sigurdson CJ. Structure of the β2-α2 loop and interspecies prion transmission. FASEB J 2012; 26:2868 - 76; http://dx.doi.org/10.1096/fj.11-200923; PMID: 22490928
- Béringue V. Prion diversity and evolution - what animal strain typing tells us. Prion 2012; 6:2 - 22
- Béringue V, Herzog L, Jaumain E, Reine F, Sibille P, Le Dur A, Vilotte JL, Laude H. Facilitated cross-species transmission of prions in extraneural tissue. Science 2012; 335:472 - 5; http://dx.doi.org/10.1126/science.1215659; PMID: 22282814
- Brown P, Gajdusek DC. Survival of scrapie virus after 3 years’ interment. Lancet 1991; 337:269 - 70; http://dx.doi.org/10.1016/0140-6736(91)90873-N; PMID: 1671114
- Smith CB, Booth CJ, Pedersen JA. Fate of prions in soil: a review. J Environ Qual 2011; 40:449 - 61; http://dx.doi.org/10.2134/jeq2010.0412; PMID: 21520752
- Wyckoff C, Vercauteren KZM. Estimating prion binding capacity of soil. Prion 2012; 6:137 - 43
- Maluquer de Motes C, Cano MJ, Torres JM, Pumarola M, Girones R. Detection and survival of prion agents in aquatic environments. Water Res 2008; 42:2465 - 72; http://dx.doi.org/10.1016/j.watres.2008.01.031; PMID: 18321558
- Health Safety Executive ACDP. The approved list of biological agents. 2004. http://www.hse.gov.uk/pubns/misc208.pdf
- Service Biosécurité et Biotechnologie. List of viruses and unconventional agents presenting at the wild state a biological risk for immunocompetent humans and/or animals and corresponding maximum biological risk. 2009.
- Clewley JP, Kelly CM, Andrews N, Vogliqi K, Mallinson G, Kaisar M, Hilton DA, Ironside JW, Edwards P, McCardle LM, et al. Prevalence of disease related prion protein in anonymous tonsil specimens in Britain: cross sectional opportunistic survey. BMJ 2009; 338:b1442; http://dx.doi.org/10.1136/bmj.b1442; PMID: 19460798
- Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, Penney M, Hegazy D, Ironside JW. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol 2004; 203:733 - 9; http://dx.doi.org/10.1002/path.1580; PMID: 15221931
- World Organisation for Animal Health. BSE: Manual of diagnostic tests and vaccines for terrestrial animals. 2010.
- de Marco MF, Linehan J, Gill ON, Clewley JP, Brandner S. Large-scale immunohistochemical examination for lymphoreticular prion protein in tonsil specimens collected in Britain. J Pathol 2010; 222:380 - 7; http://dx.doi.org/10.1002/path.2767; PMID: 20922767
- Arellano-Anaya ZE, Savistchenko J, Mathey J, Huor A, Lacroux C, Andréoletti O, Vilette D. A simple, versatile and sensitive cell-based assay for prions from various species. PLoS One 2011; 6:e20563; http://dx.doi.org/10.1371/journal.pone.0020563; PMID: 21655184
- Edgeworth JA, Jackson GS, Clarke AR, Weissmann C, Collinge J. Highly sensitive, quantitative cell-based assay for prions adsorbed to solid surfaces. Proceedings of the National Academy of Sciences 2009;Early edition:1-5.
- Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 2001; 411:810 - 3; http://dx.doi.org/10.1038/35081095; PMID: 11459061
- Caughey B, Orrù CD, Wilham JM, Vascellari S, Hyghson AG, Raymond LD, et al. Prion-seeded conversion of recombinant PrP: implications for prion biology and diagnostics. Prion 2012; 6:2 - 22
- Centers for Disease Control and Prevention (US), National Institutes of Health. Prion diseases. In: Chosewood LC, Wilson DE, editors. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. CDC; 2009. 282-289.
- CEN. Laboratory biorisk management. Report: CWA 2011; 15793:2011
- Murphy RGL, Scanga JA, Powers BE, Pilon JL, Vercauteren KC, Nash PB, Smith GC, Belk KE. Alkaline hydrolysis of mouse-adapted scrapie for inactivation and disposal of prion-positive material. J Anim Sci 2009; 87:1787 - 93; http://dx.doi.org/10.2527/jas.2008-1492; PMID: 19098230
- Taylor DM, Fernie K, McConnell I. Inactivation of the 22A strain of scrapie agent by autoclaving in sodium hydroxide. Vet Microbiol 1997; 58:87 - 91; http://dx.doi.org/10.1016/S0378-1135(97)00103-X; PMID: 9453120
- Taylor DM. Inactivation of transmissible degenerative encephalopathy agents: A review. Vet J 2000; 159:10 - 7; http://dx.doi.org/10.1053/tvjl.1999.0406; PMID: 10640408
- Fichet G, Comoy E, Duval C, Antloga K, Dehen C, Charbonnier A, McDonnell G, Brown P, Lasmézas CI, Deslys JP. Novel methods for disinfection of prion-contaminated medical devices. Lancet 2004; 364:521 - 6; http://dx.doi.org/10.1016/S0140-6736(04)16810-4; PMID: 15302195
- Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 2007; 3:e93; http://dx.doi.org/10.1371/journal.ppat.0030093; PMID: 17616973
- World Health Organisation. WHO infection control guidelines for transmissible spongiform encephalopathies. 2000. Report: WHO/CDS/CSR/APH/2000.3.
- Rutala WA, Weber DJ, Society for Healthcare Epidemiology of America. Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infect Control Hosp Epidemiol 2010; 31:107 - 17; http://dx.doi.org/10.1086/650197; PMID: 20055640
- Müller H, Stitz L, Wille H, Prusiner SB, Riesner D. Influence of water, fat, and glycerol on the mechanism of thermal prion inactivation. J Biol Chem 2007; 282:35855 - 67; http://dx.doi.org/10.1074/jbc.M706883200; PMID: 17878157
- Rogez-Kreuz C, Yousfi R, Soufflet C, Quadrio I, Yan ZX, Huyot V, Aubenque C, Destrez P, Roth K, Roberts C, et al. Inactivation of animal and human prions by hydrogen peroxide gas plasma sterilization. Infect Control Hosp Epidemiol 2009; 30:769 - 77; http://dx.doi.org/10.1086/598342; PMID: 19563265
- Edgeworth JA, Farmer M, Sicilia A, Tavares P, Beck J, Campbell T, Lowe J, Mead S, Rudge P, Collinge J, et al. Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet 2011; 377:487 - 93; http://dx.doi.org/10.1016/S0140-6736(10)62308-2; PMID: 21295339
- Bauman PA, Lawrence LA, Biesert L, Dichtelmüller H, Fabbrizzi F, Gajardo R, Gröner A, Jorquera JI, Kempf C, Kreil TR, et al. Critical factors influencing prion inactivation by sodium hydroxide. Vox Sang 2006; 91:34 - 40; http://dx.doi.org/10.1111/j.1423-0410.2006.00790.x; PMID: 16756599
- Elmoualij B, Thellin O, Gofflot S, Heinen E, Levif P, Séguin J, et al. Decontamination of prions by the flowing afterglow of a reduced-pressure N2−O2 cold-plasma. Plasma Process Polym 2012; 9:612 - 8; http://dx.doi.org/10.1002/ppap.201100194
- Lehmann S, Pastore M, Rogez-Kreuz C, Richard M, Belondrade M, Rauwel G, Durand F, Yousfi R, Criquelion J, Clayette P, et al. New hospital disinfection processes for both conventional and prion infectious agents compatible with thermosensitive medical equipment. J Hosp Infect 2009; 72:342 - 50; http://dx.doi.org/10.1016/j.jhin.2009.03.024; PMID: 19541387
- Fichet G, Antloga K, Comoy E, Deslys JP, McDonnell G. Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J Hosp Infect 2007; 67:278 - 86; http://dx.doi.org/10.1016/j.jhin.2007.08.020; PMID: 17942185
- Leunda A, Pauwels K, Herman P, Verheust C, Zorzi W, Thellin O, et al. Risk assessment of laboratories involving the manipulation of unconventional agents causing TSE. Brussels: Scientific Institute of Public Health; 2009. Report: D/2009/2505/49.
- Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. Infectivity of scrapie prions bound to a stainless steel surface. Mol Med 1999; 5:240 - 3; PMID: 10448646
- Maddison B, Baker C, Gough K, Hawkins S, Simmons H. Evaluation of pen decontamination regimes for the effective removal of environmental scrapie. Prion 2012; 6:137 - 43
- Leunda A, Roels S, Van Vaerenbergh B. Dismantlement of laboratories performing rapid detection of Transmissible Spongiform Encephalopathy. 2011. Report: ISP/41/CU/11-1034. http://www.biosafety.be/CU/PDF/Dismantlement_Lab_TSE.pdf