Abstract
In the brain, apolipoprotein E (APOE) delivers cholesterol-rich lipoproteins to neurons to support synaptogenesis and maintenance of synaptic connections. Three APOE alleles exist in the human population with ε4 being an Alzheimer disease (AD) risk gene and ε2 being protective relative to the common ε3 variant. Many hypotheses have been advanced concerning allele-specific effects of APOE on neurodegeneration including effects on Aβ clearance, synaptic transmission, or neurotoxicity. Central to most proposed APOE functions is its interaction with receptors that mediate cellular uptake of this ligand. Several members of the LDL receptor gene family have been implicated as APOE receptors in the (patho)physiology of APOE in the brain, yet their specific modes of action in AD remain controversial. Recently, the pro-neurotrophin receptor sortilin has been identified as a novel APOE receptor in neurons. Ablation of sortilin expression in mice results in accumulation of APOE and Aβ in the brain. Moreover, primary neurons lacking sortilin exhibit significantly impaired uptake of APOE/Aβ complexes. Despite increased brain APOE levels, sortilin-deficient animals recapitulate anomalies in brain lipid homeostasis seen in APOE null mice, indicating functional deficiency in APOE uptake pathways. Taken together, these findings suggest a link between Aβ catabolism and pro-neurotrophin signaling converging on this receptor pathway.
Introduction
APOE is a 299 amino acid glycoprotein that transports cholesterol and other lipids (such as phospholipids and sulfatides) as part of a lipoprotein complex in the periphery as well as in the central nervous system (CNS). APOE is expressed in several organs, with the highest expression in the liver, followed by the brain. In the brain, APOE is synthesized and secreted primarily by glia and to some extent by microglia.Citation1,Citation2 However, neurons can also produce APOE under excitotoxic injury.Citation3 Following secretion into the interstitial fluid, APOE associates with disc-like lipid particles, which are composed of phospholipids and cholesterol. Subsequently, this lipid/APOE complex is endocytosed to deliver cholesterol and other lipids to neurons.
In humans, APOE is encoded by a single gene with 3 polymorphic alleles (ε2, ε3, and ε4) that differ from one another by a single amino acid change. The APOE genotype is the major genetic risk factor for developing late-onset Alzheimer disease (LOAD), with the ε4 allele being an AD risk factor and the ε2 allele being protective.Citation4,Citation5 Several mechanisms have been suggested by which APOE exerts its isoform-specific effect on brain Aβ levels and amyloid plaque burden. Mainly it acts as a binding chaperone of Aβ thereby modulating Aβ clearance, aggregation, and/or deposition. Common to many hypotheses on the involvement of APOE in neuronal function and AD pathogenesis is the central role of APOE receptors as key regulators of APOE biology
In this review, I will discuss recent findings on APOE receptors and their important contributions to amyloid β clearance. In particular, I will focus on the roles of two established APOE receptors in the brain, called low-density lipoprotein receptor (LDLR) and the LDLR-related protein 1 (LRP1) as well as a newly described APOE receptor termed sortilin.
Low-Density Lipoprotein Receptor (LDLR) Gene Family
The main class of APOE receptors is a group of endocytic receptors called LDLR gene family or LDLR-related proteins (LRPs). These receptors are responsible for cellular uptake of APOE-containing lipoproteins in most cell types in the CNS and in peripheral tissues. Receptor-mediated uptake of APOE-containing lipoproteins serves to deliver cholesterol and other lipids essential for cellular homeostasis such as energy metabolism, membrane biosynthesis, or production of steroid hormones. The LDLR gene family comprises nine closely related surface receptors: LDLR, very-low density lipoprotein receptor (VLDLR), LRP1, LRP1b, LRP2, LRP4, LRP5, LRP6, and LRP8 (). The extracellular N terminal domain consists of a conserved arrangement of clusters of complement-type repeats and is the main ligand binding site. The other extracellular domains consist of epidermal growth factor (EGF) homology domains composed of EGF-type repeats and a β-propeller that are involved in the release of ligands in the acidic conditions of the endosomes (for review, see ref. Citation6). Their cytoplasmic tails contain one to three NPxY motifs, which serve as binding site for adaptors or scaffolding proteins controlling endocytosis and cellular recycling of the receptors. Binding of ligands, such as APOE-containing lipoproteins to the receptor at the cell surface results in internalization of receptor-ligand complexes. Ligands are discharged in lysosomes while the unliganded receptors are recycled to the cell surface to undergo the next endocytic cycle (). The functional relevance of LRPs for the organism is underscored by phenotypes seen in humans and animal models of receptor deficiencies. For instance, the LDLR represents the main pathway for clearance of cholesterol-rich lipoproteins from the circulation. Defects in LDLR function leads to an increase in serum cholesterol levels that correlates with an elevated risk of developing cardiovascular disease in patients.Citation7 In the nervous system, LRPs involved in the control of brain development and architecture (LRP2, LRP8, VLDLR), in the regulation of synaptic activity (LRP8, LRP1) and as APOE receptors in AD (LDLR, LRP1, VLDLR, LRP8) (for review see ref. Citation8).
Figure 1. Members of the LDL receptor gene family and VPS10P domain receptors or sortilins. Note that SORLA shares some structural domains with the LDL receptor gene family.
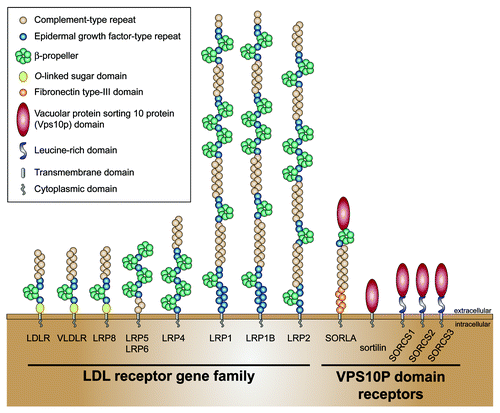
Figure 2. Schematic representation cellular APOE uptake pathways. (A) LDLR and LRP1 are classical endocytic receptors. Aβ/APOE complexes associate either with LDLR or LRP1 at the plasma membrane (step 1). Note that Aβ can directly bind to LRP1 while binding to the LDLR is mediated through association of Aβ with APOE. After endocytosis, Aβ and APOE reach the endosomal compartment (step 2) and finally the lysosomes for degradation (step 4) while the receptors are recycled to the cell surface (step 3). (B) Sortilin shows a cellular trafficking pathway that is distinct from that of LRPs. Following internalization from the cell surface (step 1) sortilin moves from the endosomal compartment to the trans-Golgi network (TGN), to continue intracellular shuttling between endocytic and secretory organelles (step 2). (C) Hypothetical model how sortilin might influence APOE3 and APOE4 trafficking in neurons. After dissociation of the Aβ/APOE complexes in the endosomes (step 2), Aβ and APOE isoforms follow different intracellular routes. Aβ is degraded in the lysosomes (step 3). APOE3 recycles back to cell surface (step 4) but APOE4 is trapped within intracellular compartments, possibly in the endosomes (step 2). Sortilin is a trafficking receptor that has a higher affinity for APOE3 compared with APOE4. Sortilin might thereby release APOE4 already in the endosomal compartment whereas it could traffic APOE3 back to the secretory pathway (step 4).
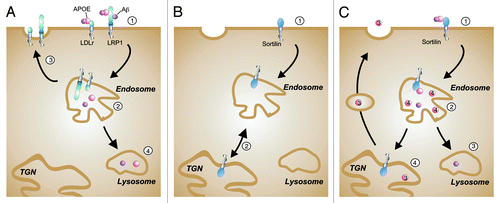
This latter role is supported by the fact that LRPs mediate the clearance of amyloid β (Aβ) bound to APOE. The two main receptors discussed in this context are LDLR and LRP1. Overexpression of the LDL receptor or of the LRP1 mini receptors reduces systemic levels of APOE in the CNS and decreases, in the case of LDLR, amyloid deposition.Citation9-Citation11 Conversely, analysis of mouse models in which Ldlr or Lrp1 have been inactivated showed increased APOE levels in the brain.Citation6 It is well described that APOE-mediated clearance of Aβ peptides can occur at different locations in the brain. Whereas the LDLR mainly mediates uptake of APOE into microglia and astrocytes, LRP1 constitutes an uptake pathway for APOE in neurons but also at the level of the brain-blood barrier (BBB).Citation12 Another difference among these two APOE receptors is their specificity to APOE isoforms. In contrast to the LDL receptor, which does not bind APOE2, LRP1 recognizes all APOE isoforms.Citation13 Also, whereas APOE is the only known ligand for the LDLR in the brain, LRP1 may have a more complex role in brain (patho)physiology since it is able to interact with numerous ligands including amyloid precursor protein (APP; the substrate from which Aβ is derived), free Aβ, as well as Aβ carrier proteins, such as APOE and α2 macroglobulin ().Citation6 Contrary to LDLR, LRP1 plays not only a role in Aβ clearance but also in APP internalization, trafficking and processing.Citation6
Sortilin, a Novel APOE Receptor with Unique Functions
Sortilin, a novel neuronal receptor pathway
While many studies have focused on the role of LRPs in brain APOE metabolism, recent studies uncovered an unexpected novel type of APOE receptor expressed in neurons, called sortilin.
Sortilin was originally purified from human brain tissue in a quest to identify new lipoprotein receptors.Citation14 The structural architecture of sortilin was unique among all mammalian surface receptors known but was later classified as part of an entire new class of type 1 receptor expressed in the nervous system called the VPS10P domain receptor family (). The structural motif common to all receptors in this family is the VPS10P domain, a 700 amino acid module in the extracellular domain that forms a 10-bladed β propeller, which is conserved from yeast to humans (; for review, see ref. Citation15). This motif represents a site for ligand binding.Citation16 Surprisingly, despite the different structure of their extracellular domains, sortilin and the LDLR gene family share common ligands including lipoprotein lipase and APOB.Citation14,Citation17-Citation19
Although sortilin predominates in neurons of the central and the peripheral nervous systems, it is also expressed in metabolic tissues including liver. Following the association of the human gene locus (SORT1) at 1p13.3 with plasma cholesterol levels and risk of myocardial infarction in several genome-wide association studies (GWASs),Citation20 sortilin was shown to facilitate hepatic lipoprotein export via binding of APOB100 in the trans-Golgi network.Citation18 As a result, sortilin ablation leads to disturbances of APOB100 trafficking, to an impaired release of very low-density lipoproteins (VLDL), and ultimately to reduced systemic cholesterol levels.Citation18 In the CNS, sortilin has a more complex role in neurotrophin signaling: it binds the pro-form of nerve growth factor (NGF; proNGF) and other neurotrophin (NT) precursors (proNTs) that induce cell death in conditions of acute or chronic stress of the nervous system.Citation21,Citation22 It also has a role in the anterograde trafficking of a class of NT receptors: the tyrosine receptor kinase (Trk receptors),Citation23 and sortilin controls the release of proNT (for review see ref. Citation15). Apart from this involvement in neurotrophin signaling, sortilin is implicated in neurodegenerative disorders. For instance, it mediates the endocytosis of progranulin (PGRN), an etiologic agent in frontotemporal lobar degeneration (FTLD), and delivers it to lysosomes for degradationCitation24 (). Thus, Sort1 ablation normalizes PGRN levels in GRN+/− mice, a model for FTLD.Citation24
Evidence that sortilin is a major neuronal APOE receptor
While the role of sortilin in lipoprotein metabolism had been documented for the liver, it came as a surprise when a similar function for the receptor in brain APOE metabolism was uncovered.Citation25 This hypothesis was mainly sparked by the findings that mice genetically deficient for the receptor have a profound increase in APOE levels. Specifically, absence of sortilin resulted in a 2-fold increase in the concentration of APOE in the hippocampus and cortex despite normal secretion of the protein from astrocytes.Citation25 The underlying molecular mechanism was traced to the ability of sortilin to bind and mediate endocytic uptake of APOE. Interestingly, sortilin interacted with all APOE isoforms but a 2-fold lower Kd for binding of APOE3 (44 nM) vs. APOE4 (114 nM) had been noticed.Citation25 Also, neurotensin, another ligand for sortilin, blocked receptor interaction with APOE3 much more efficiently than with APOE4 (80% vs. 30% inhibition, respectively). Although not fully elucidated, these findings suggest an isoform-specific interaction of sortilin with human APOE.
Together with increased APOE levels, mouse models lacking sortilin also showed increased levels of amyloid accumulation and senile plaque deposition.Citation25 The quantitative contribution of sortilin to neuronal APOE/Aβ catabolism showed that APOE-dependent uptake of Aβ was impaired in primary neurons lacking sortilin despite normal levels of LRP1,Citation25 supporting the idea that sortilin is a major receptor for catabolism of Aβ bound to APOE. In contrast to LRP1 and LDLR, sortilin expression in the CNS is restricted to neurons, arguing for a role of sortilin in neuronal Aβ catabolism.
As discussed earlier, APOE plays a key role in transport of cholesterol, phospholipids and sulfatides in the brain. An intact brain lipid metabolism is all the more relevant since dysregulation in lipid homeostasis is believed to contribute to AD pathology (for review, see ref. Citation26). One of the major consequences of altered APOE activity in the brain is the alteration of the sulfatide content. Sulfatides are sulfated galactosylceramides produced by oligodendrocytes and incorporated into the myelin sheath of axons. APOE-containing lipoproteins extract sulfatides from the myelin sheath and deliver them to neurons for uptake and catabolism via APOE receptors.Citation27 Consequently, sulfatide levels in the brain are increased in APOE-deficient but decreased in APOE overexpressing mice.Citation27 Intriguingly, sortilin-deficient mice display the very same increase in sulfatide concentrations as shown for animals lacking APOE, albeit at higher than normal levels of the apolipoprotein.Citation25,Citation27 In contrast, LRP1-deficient mice do not shown any accumulation of brain sulfatides.Citation25 Collectively, these data argue for a functional deficiency of APOE in the brain of sortilin-deficient mice.
Perspectives
Recent data document differences in intra-neuronal trafficking of APOE isoforms to underlie their distinct AD risk profiles. Following endocytic uptake by neuronal APOE receptors, APOE3 is largely re-secreted.Citation28,Citation29 In contrast, APOE4 remains trapped in endocytic compartments for an extended period of time adversely effecting neuronal functions. For instance, Chen and colleagues have shown that APOE4 (but not APOE3) reduces the neuronal surface expression of LRP8, a protein that acts as receptor both for APOE and for reelin.Citation28 Because reelin signaling protects NMDA and AMPA receptors from the harmful effects of Aβ, APOE-mediated sequestration of LRP8 in intracellular compartments critically reduces the ability of reelin to enhance synaptic glutamate receptor activity. These findings provide an alternative mechanism by which APOE4 may accelerate onset of dementia and neuronal degeneration by differentially impairing the maintenance of synaptic stability.Citation28
Yet, the receptors responsible for APOE intracellular trafficking in neurons remain to be determined. Laatsch and coworkers have shown that LRP1 plays a role in APOE trapping and recycling in a hepatoma cell lineCitation29 while in primary cortical neurons LRP8 surface expression is reduced upon APOE4 stimulation.Citation28 An exciting yet unexplored hypothesis states that sortilin may play a central role on intracellular trafficking of various APOE isoforms as well (). This hypothesis is based on the fact that, contrary to LRPs that recycle between cell surface and endosomes only, sortilin exhibits a more complex cellular trafficking pathway between plasma membrane, endosomes and the TGN. Intracellular routing of the receptor is governed by sorting motifs in the cytoplasmic tail of sortilin such as binding sites for Golgi-associated, γ-adaptin ear containing, ADP-ribosylation factor (ARF)-binding proteins (GGA) adaptor proteins that are essential for sorting target proteins between intracellular organelles.Citation30 Whether sortilin differentially affects sorting of APOE3 and APOE4 in neurons is a hypothesis that clearly warrants further investigation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by grants from the Helmholtz Association (HelMA) and the European Commission (Memories). Thomas E Willnow is acknowledged for critical reading of the manuscript and for his help in drafting the figures.
References
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta 1987; 917:148 - 61; http://dx.doi.org/10.1016/0005-2760(87)90295-5; PMID: 3539206
- Grehan S, Tse E, Taylor JM. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci 2001; 21:812 - 22; PMID: 11157067
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 2006; 26:4985 - 94; http://dx.doi.org/10.1523/JNEUROSCI.5476-05.2006; PMID: 16687490
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993; 261:921 - 3; http://dx.doi.org/10.1126/science.8346443; PMID: 8346443
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993; 90:8098 - 102; http://dx.doi.org/10.1073/pnas.90.17.8098; PMID: 8367470
- Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2012; 2:a006312; http://dx.doi.org/10.1101/cshperspect.a006312; PMID: 22393530
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232:34 - 47; http://dx.doi.org/10.1126/science.3513311; PMID: 3513311
- Herz J. Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol 2009; 20:190 - 6; http://dx.doi.org/10.1097/MOL.0b013e32832d3a10; PMID: 19433918
- Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, Mason SM, Paul SM, Holtzman DM. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron 2009; 64:632 - 44; http://dx.doi.org/10.1016/j.neuron.2009.11.013; PMID: 20005821
- Zerbinatti CV, Wahrle SE, Kim H, Cam JA, Bales K, Paul SM, Holtzman DM, Bu G. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. J Biol Chem 2006; 281:36180 - 6; http://dx.doi.org/10.1074/jbc.M604436200; PMID: 17012232
- Castellano JM, Deane R, Gottesdiener AJ, Verghese PB, Stewart FR, West T, Paoletti AC, Kasper TR, DeMattos RB, Zlokovic BV, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc Natl Acad Sci U S A 2012; 109:15502 - 7; http://dx.doi.org/10.1073/pnas.1206446109; PMID: 22927427
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008; 118:4002 - 13; http://dx.doi.org/10.1172/JCI36663; PMID: 19033669
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, et al. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res 2005; 46:1721 - 31; http://dx.doi.org/10.1194/jlr.M500114-JLR200; PMID: 15863833
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem 1997; 272:3599 - 605; http://dx.doi.org/10.1074/jbc.272.6.3599; PMID: 9013611
- Nykjaer A, Willnow TE. Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci 2012; 35:261 - 70; http://dx.doi.org/10.1016/j.tins.2012.01.003; PMID: 22341525
- Quistgaard EM, Madsen P, Grøftehauge MK, Nissen P, Petersen CM, Thirup SS. Ligands bind to Sortilin in the tunnel of a ten-bladed beta-propeller domain. Nat Struct Mol Biol 2009; 16:96 - 8; http://dx.doi.org/10.1038/nsmb.1543; PMID: 19122660
- Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem 1999; 274:8832 - 6; http://dx.doi.org/10.1074/jbc.274.13.8832; PMID: 10085125
- Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab 2010; 12:213 - 23; http://dx.doi.org/10.1016/j.cmet.2010.08.006; PMID: 20816088
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 2001; 108:779 - 84; PMID: 11560943
- Willnow TE, Kjølby M, Nykjaer A. Sortilins: new players in lipoprotein metabolism. Curr Opin Lipidol 2011; 22:79 - 85; http://dx.doi.org/10.1097/MOL.0b013e3283416f2b; PMID: 21124217
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci 2007; 10:1449 - 57; http://dx.doi.org/10.1038/nn2000; PMID: 17934455
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004; 427:843 - 8; http://dx.doi.org/10.1038/nature02319; PMID: 14985763
- Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B, Nyengaard JR, Tessarollo L, et al. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci 2011; 14:54 - 61; http://dx.doi.org/10.1038/nn.2689; PMID: 21102451
- Hu F, Padukkavidana T, Vægter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 2010; 68:654 - 67; http://dx.doi.org/10.1016/j.neuron.2010.09.034; PMID: 21092856
- Carlo AS, Gustafsen C, Mastrobuoni G, Nielsen MS, Burgert T, Hartl D, Rohe M, Nykjaer A, Herz J, Heeren J, et al. The pro-neurotrophin receptor sortilin is a major neuronal apolipoprotein E receptor for catabolism of amyloid-β peptide in the brain. J Neurosci 2013; 33:358 - 70; http://dx.doi.org/10.1523/JNEUROSCI.2425-12.2013; PMID: 23283348
- Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci 2011; 12:284 - 96; http://dx.doi.org/10.1038/nrn3012; PMID: 21448224
- Han X, Cheng H, Fryer JD, Fagan AM, Holtzman DM. Novel role for apolipoprotein E in the central nervous system. Modulation of sulfatide content. J Biol Chem 2003; 278:8043 - 51; http://dx.doi.org/10.1074/jbc.M212340200; PMID: 12501252
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A 2010; 107:12011 - 6; http://dx.doi.org/10.1073/pnas.0914984107; PMID: 20547867
- Laatsch A, Panteli M, Sornsakrin M, Hoffzimmer B, Grewal T, Heeren J. Low density lipoprotein receptor-related protein 1 dependent endosomal trapping and recycling of apolipoprotein E. PLoS One 2012; 7:e29385; http://dx.doi.org/10.1371/journal.pone.0029385; PMID: 22238606
- Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J 2001; 20:2180 - 90; http://dx.doi.org/10.1093/emboj/20.9.2180; PMID: 11331584