Abstract
One fundamental property of prions is the formation of strains—prions that have distinct biological effects, despite a common amino acid sequence. The strain phenomenon is thought to be caused by the formation of different molecular structures, each encoding for a particular biological activity. While the precise mechanism of the formation of strains is unknown, they tend to arise following environmental changes, such as passage between different species. One possible mechanism discussed here is heterogeneous seeding; the formation of a prion nucleated by a different molecular structure. While heterogeneous seeding is not the only mechanism of prion mutation, it is consistent with some observations on species adaptation and drug resistance. Heterogeneous seeding provides a useful framework to understand how prions can adapt to new environmental conditions and change biological phenotypes.
The term prion is derived from the word protein and infectious and was originally coined to describe the protein-based infectious agent found in scrapie.Citation1 Following the discovery that scrapie and the other transmissible spongiform encephelopathies (TSEs) were induced by aberrantly folded forms of the prion protein, PrP, the term prion effectively became synonymous with the TSE-causing agent.Citation2 However, the prion hypothesis proved to be useful for explaining a number of non-Mendelian genetic elements in yeastCitation3,Citation4 and fungi,Citation5 demonstrating that the principle of transmissible alternative protein conformations is extensible beyond diseases of PrP. A useful working definition of prion, from a biophysical and structural perspective, is a self-propagating aberrantly-folded protein aggregate that produces a biological effect. In the case of TSEs this effect is pathological; in Saccharomyces cerevisiae, prions manifest as non-Mendelian heritable traits; and in some fungi, prions are used for mating-type recognition.
HET-s is a prion-forming protein found in the fungus Podospora anserina, and is used in a mating-type recognition process.Citation6 This process involves two mating types; one that expresses HET-s and the other that expresses HET-S,Citation7 a non-prion-forming homolog. HET-s prions alone do not cause any biological effects, but during a mating event known as hyphal fusion, cytoplasmic mixing causes HET-s prions to come into contact with HET-S proteins, which induces a cell-death event called heterokaryon incompatability.Citation8 The prion-forming domain of HET-s, residues 218–289, is sufficient and necessary for heterokaryon incompatability.Citation9,Citation10
HET-s(218–289) forms polymorphic structures depending on the pH of the fibrillization conditions.Citation11 At physiological pH, HET-s(218–289) readily forms biologically active prions that are well ordered enough to allow for structure determination by solid state NMR (ssNMR).Citation12 The infectious prion structure is a two-rung β-solenoid (see ) that has structural complexity on par with other globularly folded β-solenoids. At low pH (~2) HET-s(218–289) forms heterogeneous non-infectious amyloid fibrils. Cryo-electron microscopy has characterized low pH amyloids whose structure appears to be related to the β-solenoid fold, but with morphological features not seen in the prion fold.Citation13 ssNMR of low pH amyloids showed spectra substantially different from those of the β-solenoid fold, indicating distinct molecular structures.Citation14 These results are not contradictory as amyloid structure can be influence by subtle preparative differences.Citation15 In previous work with low pH HET-s(218–289) we found that certain conditions cause proteolysis, with the resultant peptides forming stacked β-sheet fibrils,Citation16 similar to those formed by short amyloidogenic peptides.Citation17
Figure 1. Structural models of HET-s(218–289) polymorphs. (A) Two-rung β-solenoid (PDB ID: 2KJ3). (B) Hypothetical stacked β-sheet model.Citation21
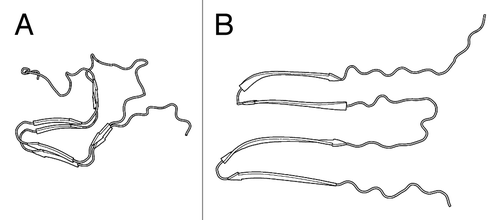
HET-s(218–289) is a useful model prion for studying the biophysical interactions of different types of amyloids. Unlike the prions of S. cerevisiae,Citation18,Citation19 HET-s(218–289) is not Q/N rich, making its amino acid composition similar to those of most other pathological amyloids. While HET-s(218–289) is smaller than the putative prion-forming domain (the proteinase K-resistant core) of PrP,Citation20 it is large enough to form a complex amyloid structure that is fundamentally irreproducible in short peptide systems.Citation17 HET-s(218–289) polymorphs are not strains in that they do not faithfully reproduce a phenotype, but this may be partly caused by the function of HET-s as a molecular trigger for a cell death process, and by evolution to fold reproducibly under physiological conditions. However, this evolved reproducible fold allows us to gain insights into the properties that differentiate prions from other amyloids, that is, the particular properties that allow for self-propagation.
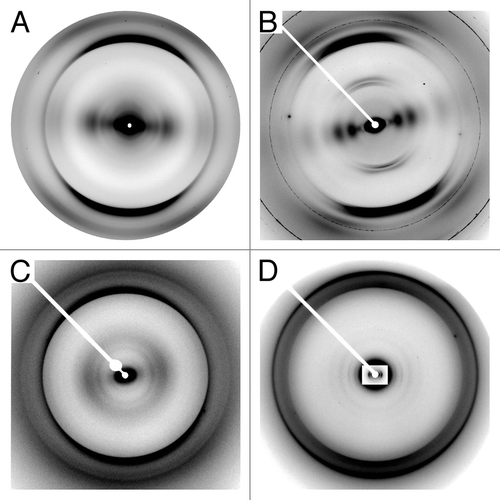
In a recent publication,Citation21 we studied polymorphs of HET-s(218–289) that formed stacked β-sheet amyloid fibrils at pH 2 without the proteolytic degradation we had previously encountered (see ).Citation16 We determined the molecular architectures of these fibrils by X-ray fiber diffraction. While fiber diffraction often does not provide enough data for high resolution structure determination, it provides directly observed data across a broad resolution range which has proved to be useful for determining important structural features.Citation22,Citation23 Fiber diffraction of stacked β-sheet polymorphs gives a 4.7Å meridional (on the axis parallel to the fiber axis) reflection (see ), caused by β-strands running perpendicular to the fiber axis—the basic property of cross-β structure.Citation21,Citation24 Equatorial (on the axis perpendicular to the fiber axis) diffraction from stacked β-sheets is dominated by an intensity maximum at ~10Å, which arises from the inter-sheet spacing. Meridional diffraction from two-rung β-solenoids also has the cross-β diffraction at 4.7Å, but has an additional 9.4Å reflection as a result of the two β-strand thick repeating unit (see ). Equatorial diffraction from β-solenoids consisted of a series of intensity maxima, indicating that the fibril is composed of a roughly cylindrical core, rather than a structure with characteristic repeats as found in stacked β-sheets.Citation21,Citation24 The fiber diffraction results were consistent with the ssNMR HET-s(218–289) β-solenoid structure, which we confirmed using ssNMR.
Figure 2. X-ray fiber diffraction patterns of HET-s(218–289) and PrP. (A) Stacked β-sheet HET-s(218–289). (B) β-solenoid HET-s(218–289). (C) rec PrP (90–231). (D) Brain-derived PrP 27–30 (strain Sc237), inset has intensity adjusted to show low-angle intensity maxima.
Using fiber diffraction to assess structure, we conducted a series of assays by seeding monomer solutions with pre-formed stacked β-sheet or β-solenoid fibrils. We found that under certain stacked β-sheet forming conditions we could propagate β-solenoid structure. However, we were unable to seed stacked β-sheet structure under β-solenoid forming conditions, indicating that the β-solenoid assembly was very robust.
To determine whether stacked β-sheets had an impact on β-solenoid fibrillization, even though they did not propagate structurally, we performed a series of fibrillization kinetics assays. Seeding assays were conducted under β-solenoid-forming conditions, with seeded assays containing fragmented pre-formed fibrils. Unseeded monomer solutions fibrillized following a sigmoidal curve with a brief initial lag phase. Solutions seeded with β-solenoid fibrils (homogeneous seeding) showed no lag phase and very fast fibrillization kinetics. Solutions seeded with stacked β-sheet fibrils (heterogeneous seeding) exhibited a unique fibrillization kinetics profile, with a rapid initial rise followed by slower rate to completion than unseeded fibrils. The final fibrils were confirmed to be β-solenoid with fiber diffraction, indicating that this unusual kinetics profile was due to an interaction between stacked β-sheet fibrils and the newly formed β-solenoids. The heterogeneously seeded fibrils were serially seeded, with each passage showing more rapid kinetics, and reproduction of homogeneously seeded profiles within three passages.
The molecular architectures of the two HET-s(218–289) polymorphs studied resemble those of recombinant PrP (recPrP) amyloids of low infectivityCitation20 and highly infectious brain-derived PrP 27–30.Citation24 Fiber diffraction from recPrP amyloid (see ) showed a characteristic stacked β-sheet pattern, similar to that shown by the stacked β-sheet HET-s(218–289) polymorph. recPrP has a repeat spacing along the fiber axis of 4.8Å, and an inter-sheet spacing across the fiber axis of 10.5Å. Fiber diffraction from brain-derived PrP 27–30 tended to be harder to interpret owing to the lipids and detergents required to purify it from brain homogenates. Certain preparations showed stronger meridional data, with reflections at 9.6Å, 6.4Å, and 4.8Å, consistent with a four β-strand repeat.Citation24 Other preparations showed less contamination on the equator, allowing for clear visualization of a series of diffraction maxima, indicating a roughly cylindrical structure (see ). The different spacing of the equatorial diffraction maxima in PrP 27–30 with respect to HET-s(218–289) is caused by the larger diameter of PrP 27–30, which can be determined by electron microscopy (EM) and X-ray diffraction.Citation11,Citation24 Fiber diffraction of PrP 27–30 showed many features consistent with a four-rung β-solenoid model derived from EM reconstructions and structural comparison,Citation25 which is similar to the two-rung β-solenoid structure of HET-s(218–289).Citation12
Concomitant with the similarities in molecular architectures, HET-s(218–289) heterogeneous seeding resembles a model of PrP adaptation. recPrP fibrils do not contain PrPSc and have very low initial infectivity,Citation26 similar to the low but not incomplete infectivity observed with low pH HET-s(218–289) polymorphs.Citation11 During serial passaging, recPrP undergoes adaptation to the new host conditions and acquires the biophysical features of PrPSc,Citation24,Citation26,Citation27 parallel to the reproduction of homogeneous seeding kinetics following serial passage of heterogeneously seeded HET-s(218–289) fibrils. The similarities between these results suggest that strain adaptation by way of changing molecular structure is a common biophysical feature of prions. Results with HET-s(218–289) show that this phenomenon can be reproduced under simple experimental conditions, and that the interaction of distinctly different molecular architectures does not need to be facilitated by cofactors. The ability for distinct architectures to interact may indicate that segments of the two conformations share structure. If this is the case, then despite the significant structural changes possible through heterogeneous seeding, the conformational space that is accessible by the initial seed could still be limited.
While heterogeneous seeding and structural mutation is a useful model for certain speciation phenomena, it is not the sole mechanism of prion strain formation. Isolation of the hyper (HY) and drowsy (DY) prion strains by passage of transmissible mink encephalopathy into Syrian hamsters likely involves heterogeneous seeding together with the presence of multiple prion strains present in the original animal.Citation28 Initial passages into hamsters resulted in reduced incubation times, consistent with structural adaptation, but on the third passage, the HY and DY strains began to diverge, with subsequent passages leading to lower incubation times until stabilization of each strain. The divergence of HY and DY may have resulted from effective isolation of two variants present in the original mink brain, but the reduction in incubation time, and in particular, the inability of HY to re-infect mink, indicate that structural mutation was present.
Heterogeneous seeding may be an underlying factor in the drug-resistance mechanisms of certain types of prions. In a recent study by Oelschlegel and Weissmann,Citation29 22L prions were propagated in a number of different cell lines with and without swainsonine (swa), an inhibitor of α-manosidase II that results in misglycosylation of PrP. They found that by serial passage of prions through cell lines in the presence of swa, they could produce swa-dependent (where presence of swa increases infectivity) prions that maintained this property even without swa present, indicating structural mutation of a fold that requires the misglycosylation to propagate efficiently. Other combinations of passaging produced swa-resistant prions that became semi-resistant after passaging without swa, indicating that the structural mutations compensated for swa, but were otherwise not optimal, and the absence of swa allowed further mutation.
In another study,Citation30 RML prions were passaged into mice treated with a therapeutic lead compound, IND24, which resulted in longer survival times of treated mice. Second passage of IND24-treated RML infected brains resulted in shorter survival times than initial infections, indicating the emergence of drug resistance. However, if IND24-treated RML infected brains were passaged without IND24 treatment, the third passage resulted in IND24-susceptible prions. The abrupt switching of IND24 resistance suggests that this mode of drug resistance may be related to selection of a resistant strain within the RML inoculum rather than structural mutation.
Heterogeneous seeding and structural mutability appear to be general biophysical phenomena that allow prions to adapt to new environmental conditions. From observations of HET-s(218–289) and PrP, we conclude that heterogeneous seeding can occur despite substantial differences in molecular architecture. However, interaction between heterogeneous structures implies that there is at least some level of similarity present, which may limit the number of conformations that can be arrived at through this mechanism. The interaction of similar structural features also extends to over-simplified model systems, such as short peptide systems, as the ability to elicit biophysical interactions only implies subtle localized structural similarities. This interplay of structural complexity and size has notably been observed in Sup35p, where the full protein shows different secondary structure from that found in the truncated Sup35 NM domain, and neither of them show the secondary structure found in the short peptide GNNQQNY.Citation31,Citation32 While heterogeneous seeding alone is not sufficient to explain all modes of prion speciation and drug resistance, it is a significant factor that can act in concert with other mechanisms, so understanding its nature is essential to understanding prion propagation.
| Abbrevations | = | PrP, prion protein |
| ssNMR | = | solid state NMR |
| recPrP | = | recombinant PrP |
| PrP 27-30 | = | proteinase K digested brain-derived PrP |
| EM | = | electron microscopy |
| HY | = | hyper prion strain |
| DY | = | drowsy prion strain |
| swa | = | swainsonine |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by US. National Institutes of Health (NIH) grants AG002132 (PI GS; Program Director Stanley Prusiner) and F31-AG040947 to WW. We thank the staff of beamline 4–2 at the Stanford Synchrotron Radiation Lightsource (SSRL) and BioCAT at the Advanced Photon Source (APS). The SSRL is a national user facility operated by Stanford University on behalf of the DOE. The SSRL Structural Molecular Biology Program is supported by DOE and NIH. The APS is supported by DOE. BioCAT is supported by NIH grant 9 P41 GM103622.
References
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136 - 44; http://dx.doi.org/10.1126/science.6801762; PMID: 6801762
- Prusiner SB. Molecular biology of prion diseases. Science 1991; 252:1515 - 22; http://dx.doi.org/10.1126/science.1675487; PMID: 1675487
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 1994; 264:566 - 9; http://dx.doi.org/10.1126/science.7909170; PMID: 7909170
- Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. [psi+] Science 1995; 268:880 - 4; http://dx.doi.org/10.1126/science.7754373; PMID: 7754373
- Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci U S A 1997; 94:9773 - 8; http://dx.doi.org/10.1073/pnas.94.18.9773; PMID: 9275200
- Saupe SJ. The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin Cell Dev Biol 2011; 22:460 - 8; http://dx.doi.org/10.1016/j.semcdb.2011.02.019; PMID: 21334447
- Greenwald J, Buhtz C, Ritter C, Kwiatkowski W, Choe S, Maddelein M-L, Ness F, Cescau S, Soragni A, Leitz D, et al. The mechanism of prion inhibition by HET-S. Mol Cell 2010; 38:889 - 99; http://dx.doi.org/10.1016/j.molcel.2010.05.019; PMID: 20620958
- Seuring C, Greenwald J, Wasmer C, Wepf R, Saupe SJ, Meier BH, Riek R. The mechanism of toxicity in HET-S/HET-s prion incompatibility. PLoS Biol 2012; 10:e1001451; http://dx.doi.org/10.1371/journal.pbio.1001451; PMID: 23300377
- Balguerie A, Dos Reis S, Ritter C, Chaignepain S, Coulary-Salin B, Forge V, Bathany K, Lascu I, Schmitter J-M, Riek R, et al. Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. EMBO J 2003; 22:2071 - 81; http://dx.doi.org/10.1093/emboj/cdg213; PMID: 12727874
- Maddelein ML, Dos Reis S, Duvezin-Caubet S, Coulary-Salin B, Saupe SJ. Amyloid aggregates of the HET-s prion protein are infectious. Proc Natl Acad Sci U S A 2002; 99:7402 - 7; http://dx.doi.org/10.1073/pnas.072199199; PMID: 12032295
- Sabaté R, Baxa U, Benkemoun L, Sánchez de Groot N, Coulary-Salin B, Maddelein ML, Malato L, Ventura S, Steven AC, Saupe SJ. Prion and non-prion amyloids of the HET-s prion forming domain. J Mol Biol 2007; 370:768 - 83; http://dx.doi.org/10.1016/j.jmb.2007.05.014; PMID: 17532341
- Van Melckebeke H, Wasmer C, Lange A, Ab E, Loquet A, Böckmann A, Meier BH. Atomic-resolution three-dimensional structure of HET-s(218-289) amyloid fibrils by solid-state NMR spectroscopy. J Am Chem Soc 2010; 132:13765 - 75; http://dx.doi.org/10.1021/ja104213j; PMID: 20828131
- Mizuno N, Baxa U, Steven AC. Structural dependence of HET-s amyloid fibril infectivity assessed by cryoelectron microscopy. Proc Natl Acad Sci U S A 2011; 108:3252 - 7; http://dx.doi.org/10.1073/pnas.1011342108; PMID: 21300906
- Wasmer C, Soragni A, Sabaté R, Lange A, Riek R, Meier BH. Infectious and noninfectious amyloids of the HET-s(218-289) prion have different NMR spectra. Angew Chem Int Ed Engl 2008; 47:5839 - 41; http://dx.doi.org/10.1002/anie.200704896; PMID: 18548467
- Petkova AT, Leapman RD, Guo Z, Yau W-M, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science 2005; 307:262 - 5; http://dx.doi.org/10.1126/science.1105850; PMID: 15653506
- Wan W, Wille H, Stöhr J, Baxa U, Prusiner SB, Stubbs G. Degradation of fungal prion HET-s(218-289) induces formation of a generic amyloid fold. Biophys J 2012; 102:2339 - 44; http://dx.doi.org/10.1016/j.bpj.2012.04.011; PMID: 22677387
- Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJW, McFarlane HT, et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 2007; 447:453 - 7; http://dx.doi.org/10.1038/nature05695; PMID: 17468747
- Kabani M, Melki R. Yeast prions assembly and propagation: contributions of the prion and non-prion moieties and the nature of assemblies. Prion 2011; 5:277 - 84; http://dx.doi.org/10.4161/pri.18070; PMID: 22052349
- Holmes DL, Lancaster AK, Lindquist S, Halfmann R. Heritable remodeling of yeast multicellularity by an environmentally responsive prion. Cell 2013; 153:153 - 65; http://dx.doi.org/10.1016/j.cell.2013.02.026; PMID: 23540696
- Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB. Synthetic mammalian prions. Science 2004; 305:673 - 6; http://dx.doi.org/10.1126/science.1100195; PMID: 15286374
- Wan W, Bian W, McDonald M, Kijac A, Wemmer DE, Stubbs G. Heterogeneous seeding of a prion structure by a generic amyloid form of the fungal prion-forming domain HET-s(218-289). J Biol Chem 2013; 288:29604 - 12; http://dx.doi.org/10.1074/jbc.M113.505511; PMID: 23986444
- Watson JD, Crick FHC. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953; 171:737 - 8; http://dx.doi.org/10.1038/171737a0; PMID: 13054692
- Franklin RE, Gosling RG. Molecular configuration in sodium thymonucleate. Nature 1953; 171:740 - 1; http://dx.doi.org/10.1038/171740a0; PMID: 13054694
- Wille H, Bian W, McDonald M, Kendall A, Colby DW, Bloch L, Ollesch J, Borovinskiy AL, Cohen FE, Prusiner SB, et al. Natural and synthetic prion structure from X-ray fiber diffraction. Proc Natl Acad Sci U S A 2009; 106:16990 - 5; http://dx.doi.org/10.1073/pnas.0909006106; PMID: 19805070
- Wille H, Michelitsch MD, Guénebaut V, Supattapone S, Serban A, Cohen FE, Agard DA, Prusiner SB. Structural studies of the scrapie prion protein by electron crystallography. Proc Natl Acad Sci U S A 2002; 99:3563 - 8; http://dx.doi.org/10.1073/pnas.052703499; PMID: 11891310
- Makarava N, Kovacs GG, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV. Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease. PLoS Pathog 2011; 7:e1002419; http://dx.doi.org/10.1371/journal.ppat.1002419; PMID: 22144901
- Makarava N, Kovacs GG, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV. Stabilization of a prion strain of synthetic origin requires multiple serial passages. J Biol Chem 2012; 287:30205 - 14; http://dx.doi.org/10.1074/jbc.M112.392985; PMID: 22807452
- Bessen RA, Marsh RF. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol 1992; 73:329 - 34; http://dx.doi.org/10.1099/0022-1317-73-2-329; PMID: 1531675
- Oelschlegel AM, Weissmann C. Acquisition of drug resistance and dependence by prions. PLoS Pathog 2013; 9:e1003158; http://dx.doi.org/10.1371/journal.ppat.1003158; PMID: 23408888
- Berry DB, Lu D, Geva M, Watts JC, Bhardwaj S, Oehler A, Renslo AR, DeArmond SJ, Prusiner SB, Giles K. Drug resistance confounding prion therapeutics. Proc Natl Acad Sci U S A 2013; 110:E4160 - 9; http://dx.doi.org/10.1073/pnas.1317164110; PMID: 24128760
- Luckgei N, Schütz AK, Bousset L, Habenstein B, Sourigues Y, Gardiennet C, Meier BH, Melki R, Böckmann A. The conformation of the prion domain of Sup35p in isolation and in the full-length protein. Angew Chem Int Ed Engl 2013; 52:12741 - 4; http://dx.doi.org/10.1002/anie.201304699; PMID: 24123863
- Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005; 435:773 - 8; http://dx.doi.org/10.1038/nature03680; PMID: 15944695
