Abstract
Prion diseases are emerging infectious disorders that affect several mammalian species including humans. The transmissible agent is comprised of PrPSc, a misfolded isoform of the normal host-encoded prion protein PrPC. Immunodetection of PrPSc is often utilized for prion disease diagnosis and tracking spread of the infectious agent through the host. We have developed a rapid, high-throughput 96 well immunoassay, which is specific for the detection of PrPSc. This assay has PrPSc detection limits similar to Western blot and is advantageous because of its comparatively shorter running time, smaller start-up and operation costs, and large sample capacity.
Introduction
Prion diseases are a group of transmissible neurodegenerative diseases that affect many animal species. The animal prion diseases include scrapie, transmissible mink encephalopathy, chronic wasting disease and bovine spongiform encephalopathy.Citation1,Citation2 The human prion diseases can be acquired, inherited or can occur sporadically and include Creutzfeldt-Jakob disease, Gerstmann-Straussler-Scheinker disease, fatal familial insomnia and kuru.Citation3 Prion diseases have long asymptomatic incubation periods from months to decades followed by a short symptomatic phase characterized by progressive cognitive and/or motor deficits.Citation3 During the asymptomatic phase, prion replication can be detected in the central nervous system and in extraneural sites.Citation4 Currently, there is no treatment available for prion diseases and they are inevitably fatal.
PrPSc, a misfolded isoform of the host-encoded prion protein PrPC, is widely distributed throughout the prion-infected host, with the highest levels nearly always occurring in the central nervous system.Citation5–Citation9 Extraneural distribution of PrPSc can include secondary lymphoreticular tissues, peripheral nervous system tissues, muscle, blood and urine.Citation4,Citation10–Citation14 The pattern of PrPSc distribution is influenced by many factors including the host species infected, route of infection, strain of agent and the length of time the host was infected.Citation15 While animal bioassay is currently the most sensitive method of detection of the prion agent, immunodetection of PrPSc is an important means of determining agent spread in prion-infected hosts.
Immunodetection of PrPSc is most often performed using immunohistochemistry and western blot analysis. Other methods of PrPSc detection include slot blot, ELISA and conformation dependent immunoassay (CDI). In the slot blot procedure, tissue homogenate is filtered through a nitrocellulose membrane using a slot blot device and PrPSc is detected with an anti-PrP antibody.Citation16 Several types of ELISAs have been developed for the detection of PrPSc from brain and lymph nodes. PrPSc is captured with an anti-PrP antibody or by other nonbiological ligands. Next, the captured PrPSc is incubated with a second anti-PrP antibody followed by incubation with an enzyme-coupled secondary antibody and detected using chemiluminescence.Citation17 CDI measures anti-PrP antibody binding to denatured and native forms of PrPSc using PrP-specific antibodies. Following denaturation, specific epitopes on PrPSc are exposed that remain hidden in the native form of the protein. This assay automatically corrects for background signals produced by PrPC.Citation18
Currently, there are several methods available to enhance PrPSc detection though concentration or amplification. A limited amount of tissue can be analyzed using the PrPSc detection techniques described above. In tissues that have a low concentration of PrPSc, these methods may fail to detect PrPSc from crude tissue homogenates. To address this limitation, PrPSc can be extracted and concentrated using sodium phosphotungstic acid precipitation (NaPTA) or detergent extraction and differential centrifugation.Citation19,Citation20 Additionally, levels PrPSc that are below the threshold of immunodetection (e.g., western blot) can be amplified to detectable levels using protein misfolding cyclic amplification, quaking-induced conversion or the amyloid seeding assay.Citation21–Citation23 These protocols concentrate or amplify small amounts of PrPSc prior to detection by the methods discussed above.
Here we report a novel, high-throughput assay for the detection of PrPSc using a 96-well plate format. This assay exhibits low levels of background signal and is highly specific for the detection of PrPSc from brain homogenate. PrPSc detection limits are comparable to those achieved using western blot analysis with several advantages including a more rapid analysis time, lower cost, and the ability to accommodate a larger number of samples in a single experiment.
Results
Specificity of the 96-well immunoassay for PrPSc detection.
The initial specificity of the 96-well immunoassay to detect PrPSc was determined using 250 µg equivalents of PK-digested HY TME-infected brain homogenate as the positive control and 250 µg equivalents of PK-digested uninfected brain homogenate or DPBS alone as the negative controls (). To determine antibody specificity, the plate was incubated with either: (1) no antibodies, (2) primary antibody alone, (3) secondary antibody alone or (4) both primary and secondary antibodies. Incubation of DPBS with no antibodies, primary antibody alone, secondary antibody alone, or with both primary and secondary antibodies yielded a relative intensity between 0.015 to 0.046 (data not shown) and is defined as the background of the assay. Incubation of PK-digested uninfected brain homogenate with no antibodies, primary antibody alone, secondary antibody alone or primary and secondary antibodies resulted in a relatively higher intensity ranging between 0.279 to 0.298 (, Wells 1–4). To reduce background signal, we incubated the wells with hydrogen peroxide in methanol prior to denaturation with guanidine thiocyanate. Hydrogen peroxide inactivates endogenous peroxidases in the brain homogenate reducing non-specific reactions in assays that utilize enzymatic reaction of horseradish peroxidase as a means of signal detection.Citation26 The addition of this step reduced the relative background intensity of uninfected brain homogenate to background levels (, Wells 5–7). Following incubation with both primary and secondary antibodies, the relative intensity of uninfected brain homogenate increased to 0.170 (, Well 8). Incubation of PK-digested HY TME PrPSc with no antibodies, primary antibody alone, or secondary antibody alone yielded a relative intensity ranging from 0.213 to 0.261 without hydrogen peroxide in methanol treatment (, Wells 9–11) and 0.017 to 0.025 with hydrogen peroxide in methanol treatment (, Wells 13–15). Following incubation with both primary and secondary antibodies, the relative intensity of PK-digested HY TME PrPSc was 1.102 without hydrogen peroxide in methanol treatment (, Well 12) and 1.0 with hydrogen peroxide in methanol treatment (, Well 16).
Comparison of PrP detection limits using western blot and 96-well immunoassay.
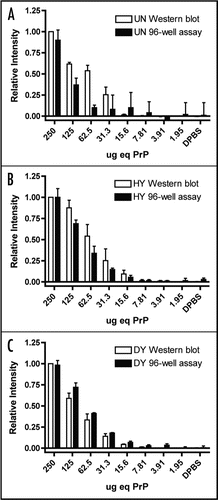
Serial 2-fold dilutions of uninfected brain homogenate were analyzed by western blot and 96-well assay and PrPC could be detected to 25.68 ± 6.29 µg equivalents by western blot and 116.01 ± 2.32 µg equivalents by 96-well immunoassay (, and ). The detection limit of PrPSc by western blot and 96-well immunoassay was determined using 2-fold serial dilutions of PK-treated HY or DY TME-infected brain homogenates ( and ) using the monoclonal anti-prion protein antibody, 3F4. HY TME PrPSc could be detected to 10.95 ± 0.66 µg equivalents by western blot and 18.22 ± 8.72 µg equivalents by 96-well immunoassay (). DY TME PrPSc could be detected to 13.30 ± 1.18 µg equivalents by western blot and 13.21 ± 1.41 µg equivalents by 96-well immunoassay ().
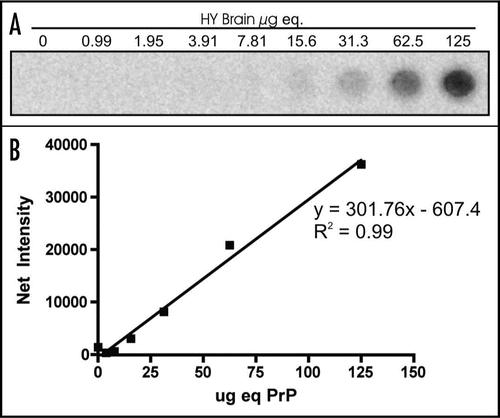
Production of a PrPSc standard curve using 96-well immunoassay.
Three replicates of a 2-fold serial dilution of PK-treated HY TME-infected brain homogenate ranging from 125 µg equivalents to 0.990 µg equivalents was used to generate a HY TME PrPSc standard curve (). The net intensity of signal produced was plotted against the µg equivalents of brain homogenate. Linear regression analysis was used to calculate the line of best fit using Prism Analysis Software ().
Discussion
We have developed a rapid, high-throughput assay for the detection of PrPSc from tissue homogenate. This 96-well immunoassay has similar PrPSc detection limits when compared to western blot analysis. HYTME PrPSc could be detected to 18.22 ± 8.72 µg equivalents by the 96-well immunoassay, which is not significantly different than the detection limit of western blot (p = 0.30). DY TME PrPSc could be detected to 13.21 ± 1.41 µg equivalents by the 96-well immunoassay, which is also not significantly different from from the detection limit of western blot (p = 0.99). It is possible to concentrate PrPSc from large volumes of brain homogenate with detergent extraction followed by differential centrifugation prior to detection by the 96-well immunoassay to increase detection limits.
There are many advantages of detecting PrPSc with the 96-well immunoassay. First, the 96-well immunoassay has a relatively short running time of approximately 5 hours. Second, a larger number of experimental samples can be analyzed at one time by using multiple 96-well plates. Samples from studies performed in 96-well plates (i.e., cell culture) can be transferred to the 96-well filter plate with binding membrane for PrPSc analysis without prior PrPSc concentration that would be necessary for western blot analysis. Due to increased capacity of the 96-well immunoassay, a PrPSc standard curve can be performed with each assay, which can be used to determine the PrPSc concentrations of unknown samples. This is extremely beneficial when analyzing a large number of samples with an unknown quantity of PrPSc. Third, the start-up and operation costs of the 96-well immunoassay are comparatively less expensive than western blot analysis since gel electrophoresis and transfer equipment are not required. Finally, the 96-well format allows for the possibility of automation that would increase the efficiency, speed and sample throughput of this immunoassay. A disadvantage of this system is that information on PrPSc migration patterns and glycoform ratios is not provided, similar to slot blot and ELISA. Overall, this assay is robust and allows for large numbers of samples to be rapidly analyzed.
Materials and Methods
Sample preparation.
Brain tissue was collected from Syrian golden hamsters infected with either the hyper (HY) or drowsy (DY) strains of transmissible mink encephalopathy (TME) agent at terminal disease or uninfected control hamsters.Citation24 Brain tissue was homogenized to 10% w/v in Dulbecco's phosphate buffered saline (DPBS; Mediatech, Herndon, VA) and was incubated with an equal volume of either proteinase K (final concentration 4 U/ml PK; Roche Diagnostics GmbH, Mannheim, Germany) at 37°C for 1 hour with constant agitation, or diluted in DPBS for undigested controls. Following incubation, the samples were 2-fold serially diluted in DPBS.
SDS-PAGE and western blot analysis.
Western blot analysis and quantification of PrPSc were performed as previously described.Citation25 Briefly, the samples were size fractioned on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P transfer membrane (Millipore, Billerica, MA). The membranes were blocked for non-specific binding with 5% w/v blotto (Bio-Rad Laboratories, Hercules, CA) in TTBS (0.05% v/v Tween-20 in tris-buffered saline) and subsequently incubated with the monoclonal anti-PrP antibody, 3F4 (1:10,000; Chemicon, Temecula, CA) in 5% blotto/TTBS followed by goat anti-mouse-horseradish peroxidase conjugated secondary antibody (1:100; Pierce, Rockford, IL) in 5% blotto/TTBS. The membranes were developed with Pierce Supersignal West Femto maximum sensitivity substrate (Pierce, Rockford, IL) according to manufacturer's instructions, imaged and quantified on a Kodak 2000R imaging station (Kodak, Rochester, NY) with a one-minute exposure time.
96-well immunoassay analysis.
Each well of the Acrowell 96 well filter plate with 0.45 µm BioTrace PVDF binding membrane (Pall Life Sciences, Ann Arbor, MI) was activated by addition of 200 µl of methanol for 30 seconds. The methanol was removed by pipette aspiration. The membrane was washed as follows: first, 200 µl of TTBS were added to each well, aspirated up and down five times by pipette and removed. This was performed a total of three times. Next, 200 µl of TTBS were added to each well and the plate was centrifuged at 1,500 xg for 1 minute in a Centra GP8R tabletop centrifuge (Thermo IEC, Waltham, MA). This was performed a total of two times. After washing, the sample was brought to 200 µl with DPBS, added to the well, and the plate was centrifuged at 1,500 xg for 1 minute. To block endogenous peroxidase activity, 200 µl of 0.3% hydrogen peroxide (Sigma, St. Louis, MO) in methanol were added to each well and the plate was incubated for 20 minutes at room temperature. The plate was centrifuged at 1,500 xg for 1 minute and washed. To denature PrPSc, 200 µl of 3 M guanidine thiocyanate (Sigma-Aldrich, St. Louis, MO) were added to each well and incubated for 10 minutes at room temperature, removed by pipette aspiration, and the plate was washed. Next, 200 µl of blocking solution (5% blotto/TTBS) were added to each well and incubated for 30 minutes at 37°C with constant agitation. The blocking solution was removed by pipette aspiration and 100 µl of the monoclonal anti-PrP antibody, 3F4 (1:10,000; Chemicon, Temecula, CA) in 5% blotto/TTBS were added. The primary antibody was incubated for 1 hour at 37°C with agitation, removed by pipette aspiration and the plate was washed. 100 µl of goat anti-mouse-horseradish peroxidase conjugated secondary antibody in 5% blotto/TTBS (1:2,000; Pierce, Rockford, IL) were added to each well and incubated for 30 minutes at 37°C with agitation, removed by pipette and the plate was washed. Next, 100 µl of Pierce Supersignal West Femto maximum sensitivity substrate were added to each well and incubated in the dark for 1 minute, centrifuged at 1,500 xg for 1 minute, and imaged on a Kodak 2000R imaging station with a 1 minute exposure time. For maximum signal detection and minimum background, reagents were prepared immediately prior to each 96-well immunoassay. Additionally, the membrane was not allowed to dry out because this would have resulted in an increase in background signal (data not shown).
Quantification of PrPSc abundance and statistical analysis.
The net intensity of PrPSc from each sample was obtained from the Kodak 1D Analysis Software. The net PrPSc intensity was standardized to a control sample containing 250 µg equivalents of PK digested brain from HY or DY TME-infected animals at terminal disease by dividing the net intensity from each sample by the net intensity from the controls. Each experiment was performed in triplicate and the average and standard deviation was calculated using Prism Analysis Software (GraphPad Software, Inc., LaJolla, CA). The relationship between the standardized PrPSc signal and µg equivalent of tissue analyzed was plotted and linear regression analysis was performed using Prism Analysis Software. The minimum µg equivalents of tissue containing detectable PrPSc were calculated from the intercept of the PrPSc regression line and the average net intensity of the DPBS control plus three times the standard deviation. Minimum level of PrPSc detection values were compared using a paired Student's t test using Prism Analysis Software.
Figures and Tables
Figure 1 96-well PrPSc immunoassay controls. (A) 96-well immunoassay and (B) quantification of brain homogenate from proteinase K digested uninfected (UN) or HY TME-agent infected (HY TME) hamsters with (+) and without (−) H2O2 in MeOH, primary antibody or secondary antibody to indicate the specificity of PrPSc immunodetection. Relative PrPSc intensity is based on well 16.
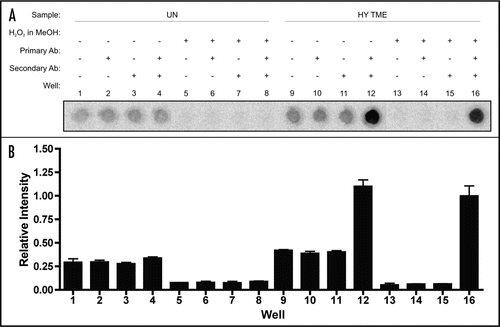
Figure 2 Limits of PrP detection using western blot analysis. Western blot analysis of two-fold serial dilutions of brain homogenates treated with (+) and without (−) proteinase K (PK) from uninfected hamsters (UN, A) or hamsters infected with the HY TME (HY) or DY TME (DY) agents (B and C, respectively).
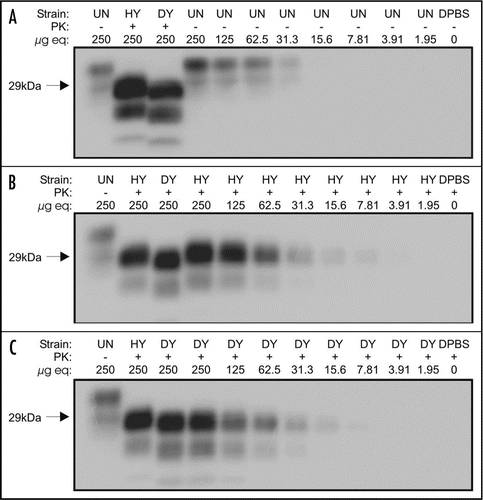
Figure 3 Limits of PrP detection using 96-well immunoassay. 96 well immunoassay of two-fold serial dilutions of brain homogenate from uninfected (UN), HY TME agent (HY) or DY TME agent (DY) infected hamsters. Samples were either proteinase K (PK) digested (+) or were left untreated (−).

Figure 4 Comparison of PrPSc detection limits by western blot and 96-well immunoassay. Quantification of PrPSc abundance of two-fold serial dilutions of uninfected (UN), HY TME-infected (HY) or DY TME-infected (DY) proteinase K digested brain material by either western blot (open bars) or 96-well immunoassay (solid bars). Represented data is the average and standard error of the mean of three independent experiments.
Acknowledgements
This work was supported by the National Center for Research Resources (P20 RR0115635-6 and C06 RR17417-01), the National Institute for Neurological Disorders and Stroke (R01 NS052609) and the National Prion Research Program (NP020041).
References
- Manson JC, Cancellotti E, Hart P, Bishop MT, Barron RM. The transmissible spongi-form encephalopathies: emerging and declining epidemics. Biochem Soc Trans 2006; 34:1155 - 1158
- Sigurdson CJ, Miller MW. Other animal prion diseases. Br Med Bull 2003; 66:199 - 212
- Goldfarb LG, Brown P. The transmissible spongiform encephalopathies. Annu Rev Med 1995; 46:57 - 65
- Eklund CM, Kennedy RC, Hadlow WJ. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis 1967; 117:15 - 22
- Bartz JC, Dejoia C, Tucker T, Kincaid AE, Bessen RA. Extraneural prion neuroinvasion without lymphoreticular system infection. J Virol 2005; 79:11858 - 11863
- Bruce ME. Agent replication dynamics in a long incubation period model of mouse scrapie. J Gen Virol 1985; 66:2517 - 2522
- Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Nat Acad Sci US 2007; 104:9741 - 9746
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136 - 144
- Prusiner SB. Molecular biology of prion diseases. Science 1991; 252:1515 - 1522
- Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. Prions in skeletal muscles of deer with chronic wasting disease. Science 2006; 311:1117
- Glatzel M, Heppner FL, Albers KM, Aguzzi A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 2001; 31:25 - 34
- Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett 2008; 582:3161 - 3166
- Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 2006; 314:133 - 136
- Mulcahy ER, Bartz JC, Kincaid AE, Bessen RA. Prion infection of skeletal muscle cells and papillae in the tongue. J Virol 2004; 78:6792 - 6798
- Dickinson AG, Outram GW. Prusiner SB, Hadlow WJ. The scrapie replication-site hypothesis and its implications for pathogenesis. Slow transmissible diseases of the central nervous system 1979; New York Academic Press 13 - 31
- Winklhofer KF, Hartl FU, Tatzelt J. A sensitive filter retention assay for the detection of PrP(Sc) and the screening of anti-prion compounds. FEBS Lett 2001; 503:41 - 45
- Creminon C, Grassi J. Lehmann S, Grassi J. Characterization of anti-PrP antibodies and measurement of PrP using ELISA techniques. Techniques in Prion Research 2004; Basel Birkhauser Verlag 117 - 131
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 1998; 4:1157 - 1165
- Bolton DC, Bendheim PE. A modified host protein model of scrapie. Ciba Found Symp 1988; 135:164 - 181
- Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 2001; 358:171 - 180
- Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods 2008; 5:211 - 212
- Castilla J, Saa P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell 2005; 121:195 - 206
- Colby DW, Zhang Q, Wang S, Groth D, Legname G, Riesner D, Prusiner SB. Prion detection by an amyloid seeding assay. Proc Natl Acad Sci USA 2007; 104:20914 - 20919
- Bessen RA, Marsh RF. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol 1992; 73:329 - 334
- Bartz JC, Kramer ML, Sheehan MH, Hutter JA, Ayers JI, Bessen RA, Kincaid AE. Prion interference is due to a reduction in strain-specific PrPSc levels. J Virol 2007; 81:689 - 697
- Magi B, Bini L, Liberatori S, Raggiaschi R, Pallini V. Walker JM. Immunoblotting of D-D electrophoresis separated proteins. The Protein Protocols Handbook 2002; Totowa Humanna Press 215 - 230