Abstract
β-2 microglobulin (β2m) is the protein responsible for amyloid deposition in Dialysis-Related Amyloidosis (DRA). Aggregation can be induced by various solution conditions including exposure to divalent metal, incubation at acidic pH, and limited proteolysis. Using Cu2+ as a trigger, we have trapped, isolated, and crystallized a stable oligomer of β2m that is populated under amyloidogenic solution conditions (Calabrese et al Nat. Struct. Mol. Biol. 15, p965-971 2008). This structure reveals that Cu2+-binding is associated with dramatic conformational rearrangements. This has allowed us to postulate a set of structural changes common to all β2m aggregation pathways. Cu2+ serves as a potential trigger in other aggregation systems such as Aβ, α-synuclein, and mammalian Prion (PrP). A comparison of Cu2+ binding to β2m and PrP reveals common features. Therefore, in addition to providing insight into DRA, induction of structure by Cu2+ binding appears to be a recurring structural motif for pathological changes in conformation.
Background
β2m is the 99 residue, 12 kDa non-covalent light chain of the Class I Major Histocompatibility Complex (MHC). Its function is to ensure proper folding and cell-surface expression of the MHC.Citation1 In the course of normal turnover, β2m is released to the serum and catabolized by the kidneys. In patients suffering from end-stage renal disease who are treated with hemodialysis therapy, β2m levels rise and amyloid plaques develop throughout the joints and connective tissue.Citation2 Although an elevated serum level of β2m may be necessary for aggregation, it is not sufficient as β2m remains stably folded at > 1 mM concentration at 37 °C.Citation3,Citation4 Furthermore, it can be reversibly folded in vitroCitation5–Citation7 and amyloid is not reported in other diseases with elevated levels in serum.Citation8,Citation9 Therefore, aggregation must be triggered by features unique to hemodialysis therapy.
As β2m exists as a stable monomer under near physiological conditions,Citation3,Citation10 much effort has been focused on understanding the molecular mechanism by which self association is triggered. Many approaches have been used to induce β2m aggregation. These include limited proteolysis,Citation11 incubation at acidic pH,Citation12 and exposure to detergents or organic solvents.Citation13,Citation14 In 2001, we discovered that β2m is a Cu2+-binding protein.Citation4 Whereas β2mapo is not amyloidogenic, β2mholo is aggregation-prone.
Cu2+ Mediated Oligomerization
We are interested in Cu2+-dependent aggregation of β2m for several reasons. First, hemodialysis patients may be exposed to elevated levels of divalent cation as a consequence of therapy. This is apparent in the historical use of Cu2+ in the preparation of dialysis membranes, and in water quality standards which permit as much as 1.6 µM Cu2+ in hemodialysate.Citation15 This is comparable to the ∼3 µM affinity of β2m measured in vitro.Citation4 Second, early experiments revealed that although Cu2+ is necessary to initiate aggregation, mature fibers remain stable in the presence of a metal chelate.Citation10 This allowed the possibility that Cu2+ action is transient and that periodic exposure to Cu2+ during dialysis therapy may be sufficient to induce aggregation. Finally, the use of an amyloidogenic trigger that operates through a defined binding event provides an opportunity for particularly clear insight into the structural rearrangements which may permit self-association.
β2m binds Cu2+ selectively over other divalents including Ca2+. Zn2+ and Ni2+ also bind, but with much weaker affinities.Citation4,Citation16 Importantly, the binding of Cu2+ but not Ni2+ leads to self-association.Citation10 We have previously asserted that at least three modes of Cu2+-binding may exist for β2m. One is based on coordination to non-native states and is linked to protein destabilization.Citation16 A second involves Cu2+-bound to the monomer in an initial capture complex, and a third represents Cu2+ bound to oligomeric states. Previous studies have implicated His13, His31, and His51 as well as the N-terminus in native-state metal binding.Citation16–Citation19 Of the three histidines above, our own studies have shown that only mutation of His31 affects native-state Cu2+ affinity.Citation16 Continued incubation under native conditions leads to aggregation that includes amyloid formation over a period of days-to-weeks.Citation4
β2m binds Cu2+ rapidly (<1 min) upon exposure. This is followed by oligomerization which completes on the hour timescale, well before mature aggregates are detected. The oligomerization rate is insensitive to the concentrations of either protein or Cu2+. This led to the suggestion that oligomerization is rate limited by a conformational rearrangement from the native to an ‘activated’ monomeric state which we termed ‘M*’.Citation10,Citation20 Another state, termed IT, identified by refolding studies may be related to this structure.Citation6,Citation21 Oligomers are native-like by circular dichroism and 1H NMR yet at the same time exhibit characteristics of amyloid as evidenced by the fluorescence response of a histological dye, Thioflavin T.Citation22 This supports a model for β2m oligomers where the global fold of β2m is maintained.Citation10
Transient Cu2+ Dependence
Although early β2m oligomers require bound Cu2+ for stability, mature fibers do not.Citation10,Citation23 The transition from chelate-sensitive to chelate-resistant states occurs gradually on the days timescale under conditions where β2m is not globally destabilized.Citation23,Citation24 The first states to exhibit resistance to chelate are not mature fibers themselves but rather are small oligomeric states dimeric-hexameric in size. These states, termed ‘chelate-resistant oligomers’, possess remarkable kinetic stability dissociating only on the days-weeks timescale and can exist without bound metal. In addition, these oligomers show partial resistance even to heating in SDS (Calabrese MF, unpublished results). As yet, a structural explanation for this stability has not been determined.
Pre-amyloid Structures
To fully understand the perturbations of β2m required for oligomerization, it is essential to determine how and where metal is coordinated. Furthermore, the consequences of metal binding enable the formation of intermolecular contacts. A structural investigation of the Cu2+-bound oligomer provides insight into both the binding site and interfaces.
Attempts to isolate and crystallize the Cu2+ bound oligomer using WT protein is complicated by the heterogeneous (monomer, dimer, tetramer, hexamer) and dynamic nature of the oligomerization reaction and its downstream conversion to aggregates.Citation10 We therefore employed mutagenesis to generate a more uniform population. Mutations were targeted based on predicted interfacesCitation20 and a single point mutant, H13F, was sufficient to increase both the extent and homogeneity of the oligomerization reaction, resulting in efficient formation of a hexamer. Nevertheless, the global stability, Cu2+ affinity, and rate of oligomerization remain similar for H13F and WT β2m.Citation16,Citation25
The hexamer is organized as a closed ring surrounding a solvent-filled central channel. The ring is three-fold symmetric demonstrating the existence of two distinct classes of interface (). Within each hexamer, six Cu2+ atoms are bound consistent with the binding stoichiometry observed in solution.Citation23,Citation25 However, contrary to our initial expectation,Citation10 metal does not localize to interfaces and does not bridge adjacent subunits. Rather, Cu2+ binding is entirely intramolecular where all coordinating ligands are derived from a single polypeptide chain. Investigation of the Cu2+-binding site reveals a roughly square-planar geometry comprised of an imidazole ring from His31 together with two amides and a carbonyl derived from the peptide backbone (). Interestingly, this site bears a striking resemblance to a previously observed Cu2+ coordination in the octapeptide region of PrP ().Citation26
Our work suggests the presence of several structurally related binding sites. Initially, Cu2+ is captured by monomeric β2m with minimal perturbation to the apo structure. This is followed by binding to reversible oligomeric species. Ultimately, Cu2+ is released from a binding site weakened by conformational changes associated with irreversible aggregation. The structure we have recently reported describes the Cu2+ binding site within the context of an oligomer.Citation25 Formation of this site requires disruption of the first β-strand and local rearrangements proximal to His31. This includes rotation of Phe30 from the hydrophobic core to face solvent, and the cis-trans isomerization of Pro32. These rearrangements cannot be accommodated in isolation, and require a set of compensatory rearrangements that propagate across the molecule (). In all, within the context of the hexamer, 26 of the 99 residues of β2m show large deviation (> 2 Å rmsd) from the position in the apo protein. Cu2+ binding may direct some but not all of these rearrangements. The energy of oligomerization may additionally stabilize alterations.
Summary
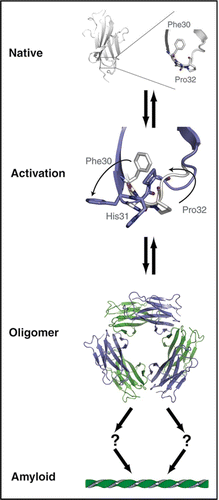
The conversion of soluble protein to amyloid plaques requires structural perturbations to a protein's native state. For β2m, these perturbations can be triggered by covalent modification or altered solution conditions including exposure to Cu2+. Using Cu2+ as a tool, we have gained unique insight into the detailed changes that initiate self association (). Most striking, the trans conformation at Pro32 and the dramatic rotation of Phe30 from the hydrophobic core toward solvent appear to be a critical switch enabling aggregation. This rearrangement may occur in β2m refolding,Citation5,Citation6 proteolytic processing,Citation11 and acid-induced restructuring.Citation27 This suggests that all β2m aggregation pathways converge at an activated state similar to M*. Indeed, an M*-like state can be mimicked by mutation of Pro32.Citation5,Citation6,Citation20 The atomic structure of one such mutant, P32A, reveals a set of rearrangements that are highly similar to those observed in the Cu2+-bound hexamer.Citation20,Citation25
In addition to elucidating common themes in β2m aggregation, several gross similarities to PrP are apparent. PrP binds Cu2+ in vivoCitation28 and this binding has been suggested to play a role in functions including metal homeostasis and response to oxidative stress.Citation29,Citation30 Although no such assertion has been made for β2m, it is intriguing to note that Cu2+ binding is specific, and the coordinating histidine and many of the residues that rearrange upon metal binding are conserved.Citation16 For both β2m and PrP, metal binding can occur in several possible geometries leading to protein destabilization, structural rearrangements, and self-association.Citation4,Citation10,Citation16,Citation30–Citation32 For PrP, multiple modes of metal binding have been determined using electron paramagnetic resonance spectroscopy.Citation30 For β2m the presence of unique binding modes has been inferred based on the ability of β2m to bind Cu2+ under both native and destabilizing conditions as well as changes in metal affinity and chelate sensitivity during the course of aggregation.Citation16,Citation20,Citation25
At least one binding mode for the octapeptide region of PrP bears a strong resemblance to the Cu2+ site in the β2m hexamer ().Citation25,Citation26 Although PrP binds Cu2+ in vivo and undergoes alterations in structure, the role of metal binding in both function and aggregation remains unclear. For example, one report suggests that Cu2+-binding promotes protease-resistance as seen in disease strainsCitation33 while others indicate that exposure to Cu2+ inhibits aggregation.Citation34 These reports, although apparently contradictory in nature, may result from the structural diversity that can result from different modes of metal-interactions.Citation35 In addition, PrP, like β2m, may have a set of closely related mechanisms for the induction of pathological aggregation with metal cations acting to bias the sampling of alternative states. The ability of metal to perturb structure appears to be a general theme in protein aggregation. Recent work suggests that self-association in diverse systems including Aβ,Citation36–Citation38 α-synuclein,Citation39,Citation40 immunoglobulin light chainCitation41, and superoxide dismutaseCitation42 may all be modulated by metal. A more thorough understanding of the structural consequences of Cu2+ binding in systems such as β2m may therefore provide broader insight into both function and disease associated with aggregation.
Abbreviations
| β2m | = | β-2 microglobulin |
| PrP | = | prion |
| MHC | = | major histocompatibility complex |
| DRA | = | dialysis-related amyloidosis |
Figures and Tables
Figure 1 Oligomeric interfaces of β2m. The β2m hexamer is shown as a semi-transparent surface flanked by surface cut-away views of each interface. In the center panel, chains are alternately colored blue and green. This illustrates the two types of interface present. In the left and right panels, one chain from each interface is represented as a solvent accessible surface, the other as sticks. Orange and red correspond to contact residues that have moved <2 Å or >2 Å r.m.s. respectively relative to the same residues in WTapo structure, PDB code 2CLR.Citation43 For the residues that have moved >2 Å r.m.s., side chains of WTapo are also drawn (blue) with their position in hexameric β2mholo indicated by arrows.
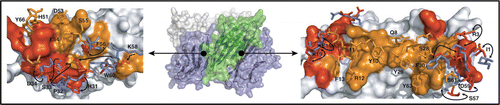
Figure 2 Cu2+ binding to β2m and PrP. (A) Cu2+-site derived from the Cu2+-bound β2m hexamer (PDB 3CIQ)Citation25 and (B) Cu2+-site derived from the HGGGW fragment of the PrP octapeptide repeat.Citation26 Both sites display coordination by an imidazole ring, two backbone nitrogens and a backbone carbonyl in a square-planar arrangement. Note that the PrP structure also contains an axial water ligand which was omitted from this figure.
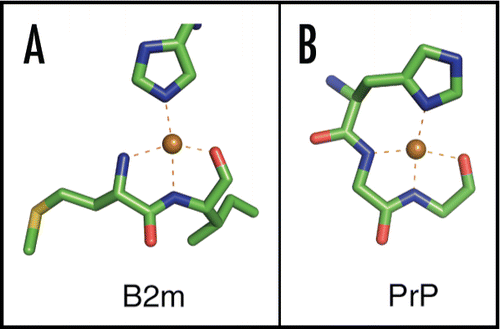
Figure 3 Unified model of β2m amyloid formation: Under physiological conditions, β2m exists as a stable, well folded monomer (upper panel, PDB 2CLRCitation43) characterized in part by a conserved cis proline at residue 32. Upon exposure to a variety of amyloigenic triggers the native structure is perturbed and amyloid formation commences. We have shown (Cu2+ or P32X mutation) or conjectured (limited proteolysis or acidic pH) that this transition involves rotation of Phe30 from the hydrophobic core toward solvent and the cis-trans isomerization of Pro32.Citation20,Citation25 We postulate that all pathways converge on a state resembling the activated monomer (M*) in which broad rearrangements occur to compensate for the cavity left by movement of Phe30. These rearrangements precede oligomerization which terminates in a hexamer.Citation10,Citation20,Citation25 The path from oligomer to mature aggregate is not known, however, as the hexamer represents a closed state, aggregation likely requires a ring-breaking event.
Acknowledgements
We thank D.V. Blaho for helpful comments. This work is supported by NIH DK54899.
Commentary to:
References
- York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol 1996; 14:369 - 396
- Floege J, Ehlerding G. Beta-2-microglobulin-associated amyloidosis. Nephron 1996; 72:9 - 26
- Okon M, Bray P, Vucelic D. 1H NMR assignments and secondary structure of human beta 2-microglobulin in solution. Biochemistry 1992; 31:8906 - 8915
- Morgan CJ, Gelfand M, Atreya C, Miranker AD. Kidney dialysis-associated amyloidosis: a molecular role for copper in fiber formation. J Mol Biol 2001; 309:339 - 345
- Kameda A, Hoshino M, Higurashi T, Takahashi S, Naiki H, Goto Y. Nuclear magnetic resonance characterization of the refolding intermediate of beta(2)-microglobulin trapped by non-native prolyl peptide bond. J Mol Biol 2005; 348:383 - 397
- Jahn TR, Parker MJ, Homans SW, Radford SE. Amyloid formation under physiological conditions proceeds via a native-like folding intermediate. Nat Struct Mol Biol 2006; 13:195 - 201
- Chiti F, Mangione P, Andreola A, Giorgetti S, Stefani M, Dobson CM, et al. Detection of two partially structured species in the folding process of the amyloidogenic protein beta 2-microglobulin. J Mol Biol 2001; 307:379 - 391
- Malaguarnera M, Restuccia S, Di Fazio I, Zoccolo AM, Trovato BA, Pistone G. Serum beta2-microglobulin in chronic hepatitis C. Dig Dis Sci 1997; 42:762 - 766
- Keating MJ. Chronic lymphocytic leukemia. Semin Oncol 1999; 26:107 - 114
- Eakin CM, Attenello FJ, Morgan CJ, Miranker AD. Oligomeric assembly of native-like precursors precedes amyloid formation by beta-2 microglobulin. Biochemistry 2004; 43:7808 - 7815
- Esposito G, Michelutti R, Verdone G, Viglino P, Hernandez H, Robinson CV, et al. Removal of the N-terminal hexapeptide from human beta2-microglobulin facilitates protein aggregation and fibril formation. Protein Sci 2000; 9:831 - 845
- McParland VJ, Kad NM, Kalverda AP, Brown A, Kirwin-Jones P, Hunter MG, et al. Partially unfolded states of beta(2)-microglobulin and amyloid formation in vitro. Biochemistry 2000; 39:8735 - 8746
- Yamamoto S, Hasegawa K, Yamaguchi I, Tsutsumi S, Kardos J, Goto Y, et al. Low concentrations of sodium dodecyl sulfate induce the extension of beta(2)-microglobulinrelated amyloid fibrils at a neutral pH. Biochemistry 2004; 43:11075 - 11082
- Yamamoto S, Yamaguchi I, Hasegawa K, Tsutsumi S, Goto Y, Gejyo F, et al. Glycosaminoglycans enhance the trifluoroethanol-induced extension of beta 2-microglobulin-related amyloid fibrils at a neutral pH. J Am Soc Nephrol 2004; 15:126 - 133
- Vorbeck-Meister I, Sommer R, Vorbeck F, Horl WH. Quality of water used for haemodialysis: bacteriological and chemical parameters. Nephrol Dial Transplant 1999; 14:666 - 675
- Eakin CM, Knight JD, Morgan CJ, Gelfand MA, Miranker AD. Formation of a copper specific binding site in non-native states of beta-2-microglobulin. Biochemistry 2002; 41:10646 - 10656
- Verdone G, Corazza A, Viglino P, Pettirossi F, Giorgetti S, Mangione P, et al. The solution structure of human beta2-microglobulin reveals the prodromes of its amyloid transition. Protein Sci 2002; 11:487 - 499
- Villanueva J, Hoshino M, Katou H, Kardos J, Hasegawa K, Naiki H, et al. Increase in the conformational flexibility of beta 2-microglobulin upon copper binding: a possible role for copper in dialysis-related amyloidosis. Protein Sci 2004; 13:797 - 809
- Lim J, Vachet RW. Using mass spectrometry to study copper-protein binding under native and non-native conditions: beta-2-microglobulin. Anal Chem 2004; 76:3498 - 3504
- Eakin CM, Berman AJ, Miranker AD. A native to amyloidogenic transition regulated by a backbone trigger. Nat Struct Mol Biol 2006; 13:202 - 208
- Dobson CM. An accidental breach of a protein's natural defenses. Nat Struct Mol Biol 2006; 13:295 - 297
- LeVine H 3rd. Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 1993; 2:404 - 410
- Calabrese MF, Miranker AD. Formation of a stable oligomer of beta-2 microglobulin requires only transient encounter with Cu(II). J Mol Biol 2007; 367:1 - 7
- Antwi K, Mahar M, Srikanth R, Olbris MR, Tyson JF, Vachet RW. Cu(II) organizes {beta}-2-microglobulin oligomers but is released upon amyloid formation. Protein Sci 2008; 17:748 - 759
- Calabrese MF, Eakin CM, Wang JM, Miranker AD. A regulatable switch mediates self-association in an immunoglobulin fold. Nat Struct Mol Biol 2008; 15:965 - 971
- Burns CS, Aronoff-Spencer E, Dunham CM, Lario P, Avdievich NI, Antholine WE, et al. Molecular features of the copper binding sites in the octarepeat domain of the prion protein. Biochemistry 2002; 41:3991 - 4001
- McParland VJ, Kalverda AP, Homans SW, Radford SE. Structural properties of an amyloid precursor of beta(2)-microglobulin. Nat Struct Biol 2002; 9:326 - 331
- Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, et al. The cellular prion protein binds copper in vivo. Nature 1997; 390:684 - 687
- Brown DR, Schmidt B, Kretzschmar HA. Effects of copper on survival of prion protein knockout neurons and glia. J Neurochem 1998; 70:1686 - 1693
- Millhauser GL. Copper and the prion protein: methods, structures, function, and disease. Annu Rev Phys Chem 2007; 58:299 - 320
- Viles JH, Klewpatinond M, Nadal RC. Copper and the structural biology of the prion protein. Biochem Soc Trans 2008; 36:1288 - 1292
- Kenward AG, Bartolotti LJ, Burns CS. Copper and zinc promote interactions between membrane-anchored peptides of the metal binding domain of the prion protein. Biochemistry 2007; 46:4261 - 4271
- Wadsworth JD, Hill AF, Joiner S, Jackson GS, Clarke AR, Collinge J. Strain-specific prion-protein conformation determined by metal ions. Nat Cell Biol 1999; 1:55 - 59
- Bocharova OV, Breydo L, Salnikov VV, Baskakov IV. Copper(II) inhibits in vitro conversion of prion protein into amyloid fibrils. Biochemistry 2005; 44:6776 - 6787
- Davies P, Brown DR. The chemistry of copper binding to PrP: is there sufficient evidence to elucidate a role for copper in protein function?. Biochem J 2008; 410:237 - 244
- Cherny RA, Legg JT, McLean CA, Fairlie DP, Huang X, Atwood CS, et al. Aqueous dissolution of Alzheimer's disease Abeta amyloid deposits by biometal depletion. J Biol Chem 1999; 274:23223 - 23228
- Bush AI, Tanzi RE. The galvanization of beta-amyloid in Alzheimer's disease. Proc Natl Acad Sci USA 2002; 99:7317 - 7319
- Miura T, Suzuki K, Kohata N, Takeuchi H. Metal binding modes of Alzheimer's amyloid beta-peptide in insoluble aggregates and soluble complexes. Biochemistry 2000; 39:7024 - 7031
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem 2001; 276:44284 - 44296
- Lee JC, Gray HB, Winkler JR. Copper(II) Binding to alpha-Synuclein, the Parkinson's Protein. J Am Chem Soc 2008;
- Davis DP, Gallo G, Vogen SM, Dul JL, Sciarretta KL, Kumar A, et al. Both the environment and somatic mutations govern the aggregation pathway of pathogenic immunoglobulin light chain. J Mol Biol 2001; 313:1021 - 1034
- Sayre LM, Perry G, Smith MA. Redox metals and neurodegenerative disease. Curr Opin Chem Biol 1999; 3:220 - 225
- Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 1987; 329:506 - 512