Abstract
The host encoded cellular prion protein (PrPC) is an N-linked glycoprotein tethered to the cell membrane by a glycophosphatidylinositol (GPI) anchor. Under certain conditions, PrPC can undergo conversion into a conformationally-altered isoform (PrPSc) widely believed to be the pathogenic agent of transmissible spongiform encephalopathies (TSEs). Understanding the tissue-specific expression of PrPC is crucial considering that cells expressing high levels of PrPC bear a risk for conversion and accumulation of PrPSc. In the present study, fifteen bovine somatic tissues were analyzed for PrPC expression by quantitative western blot and immunohistochemistry. Quantitative western blot analysis revealed highest expression of PrPC in cerebellum, obex and spinal cord. Intermediate levels were detected in thymus, intestine, nerve, heart and spleen, and lower levels in lung, muscle, kidney, lymph node, skin, pancreas and liver. Immunohistochemical analysis detected intense cellular-specific PrPC staining in neurons, thymocytes and lymphocytes. PrPC was also detected in the enteric wall, pancreatic islets of langerhans, myocardium, pulmonary alveolar sacs, renal glomeruli and dermal epithelial cells. This study demonstrated the quantitatively varied, wide-spread, tissue- and cell-specific expression pattern of PrPC in bovine somatic tissues. The importance of this study is to lay the foundation for understanding the tissue-specific expression of PrPC and to consider the potential participation of more bovine tissues in the transmission of BSE infection.
Introduction
The cellular prion protein (PrPC) is a 250-amino acid glycoprotein commonly found attached by a glycosylphosphotidylinositol (GPI) anchor to lipid rafts in the plasma membrane.Citation1 Through a poorly understood process, PrPC can undergo post-transcriptional conversion from a predominantly a-helical structure to a mainly β-sheet isoform (PrPSc).Citation2,Citation3 Substantial evidence indicates that PrPSc is the principal if not the only component of the agent causing TSEs.Citation4–Citation6 This group of neurodegenerative and infectious diseases affects several mammalian species including sheep (scrapie, the prototypical prion disease), humans (CJD and Kuru), deer (chronic wasting disease; CWD) and cattle (BSE).Citation5,Citation7 Although much is known about the role of PrPSc in TSEs, the normal function of PrPC remains enigmatic. One line of investigation proposes that PrPC is an antioxidant that directly or indirectly promotes detoxification of reactive oxygen species.Citation8 Another hypothesis proposes that PrPC has cytoprotective activity that blocks internal or environmental factors that initiate apoptosis.Citation9 In addition, several authors have proposed that PrPC participates in transmembrane signaling processes associated with cellular survival, replication and differentiation.Citation10–Citation12
Expression of endogenous PrPC is essential for TSE pathogenesis. The requisite role of PrPC in TSE pathogenesis has been unequivocally demonstrated in experiments using PrPC knockout mice. PrP null mice (Prnp0/0) do not express PrPC and are completely resistant to experimental TSE infection.Citation6 Hemizygous Prnp mice (Prnp0/+) express roughly half the PrPC of their wild-type counterparts.Citation13 The interval from experimental TSE exposure to the onset of disease is nearly twice as long in hemizygous (170 days) vs wild-type (290 days) mice. Overexpression of PrPC in transgenic mice leads to a shortening in the interval from TSE exposure to the onset of disease, with the degree of shortening proportional to the level of overexpression.Citation14 Thus, PrPC expression level, at least on a whole-animal basis, is a critical determinant of susceptibility to prion disease.
Comprehensive analyses of PrPC distribution in various somatic tissues have been reported for hamsters,Citation15 miceCitation16 and sheep.Citation17 These studies revealed that PrPC is expressed in a wide range of tissues throughout the body. However, the level at PrPC expressed was shown to vary widely between tissues.Citation15,Citation16 For example, brain and lymphoid tissues expressed relatively high levels of PrPC, while expression in liver was comparatively less.Citation16,Citation17 In TSE-infected animals, infectious PrPSc is generally found in tissues that express high endogenous levels of PrPC but not in tissues that express PrPC at lower levels.Citation7 Tissues that express relatively high levels of endogenous PrPC are thus candidates for infection by TSEs. Furthermore, knowledge of qualitative and quantitative patterns of PrPC expression may assist in understanding the role and function of PrPC in healthy animals.
The literature on distribution and expression level of PrPC protein in bovine tissues is sparse. While one study has characterized expression of PrPC mRNA in cattle,Citation18 no corresponding studies have been reported for the PrPC protein. This is surprising in light of agricultural importance of BSE and its transmissibility to humans in the form of new, variant CJD (nvCJD).Citation19 Quantification of PrPC mRNA indicated concordance with PrPC protein levels in the central nervous system (CNS), thymus, lung, kidney and muscle; whereas, no association was observed in lymph node, spleen and liver.Citation18 A comprehensive assessment of endogenous and tissue-specific expression pattern of PrPC is crucial in identifying tissue types that may be at risk for BSE infection and transmission. In the present study we sought to analyze and compare expression of PrPC in fifteen bovine somatic tissues by quantitative western blot and immunohistochemistry. Computerized quantification of western blot bands showed that PrPC was expressed in all tissues sampled. PrPC was highly expressed in CNS and thymus and expressed at lower levels in the other tissues examined. Specific antibody staining revealed that PrPC was expressed in a cell-specific manner in neurons, thymocytes, lymphocytes, keratinocytes, cardiomiocytes and pneumocytes. Wide spread expression of PrPC in bovine tissues suggests an important biological function for this molecule and expands the variety of tissues that may be at potential risk for infection and transmission of PrPSc.
Results
Western blot.
Independent assays comparing the relative levels of PrPC expression were performed on tissues from three different animals by western blot. PrPC was detected in all tissue samples in this analysis. PrPC showed three distinct migration bands corresponding to molecular weights of 35, 28 and 25 kDa (). Although we did not specifically analyze bands for glycosylation, the 35, 28 and 25 kDa bands are likely associated with the di-, mono- and un-glycosylated forms of PrPC, respectively.Citation20 The presumptive di-glycosylated form (35 kDa) was the predominant form across all tissues, whereas the presumptive mono- (28 kDa) and un-glycosylated (25 kDa) bands showed more variable intensities. The 35 kDa band dominated in CNS tissues (obex, cerebellum and spinal cord) and thymus, whereas the same form was weakly expressed in pancreas and liver. In peripheral nerve, intestine, lung and heart the 35 kDa band was observed as a doublet. The 25 kDa band was undetectable in peripheral nerve and lymphatic tissues (lymph node and thymus). Immunoreactive GAPDH was observed at 30 kDa (). Computerized quantification of PrPC bands revealed the highest (p < 0.05) level of PrPC expression in the cerebellum, obex and spinal cord (). Intermediate PrPC expression levels were found in thymus. Intestine, nerve, heart and spleen showed lower (p < 0.05) levels compared to CNS tissues, however, the lowest (p < 0.05) expression levels were observed in lung, muscle, kidney, lymph node, skin, pancreas and liver ().
Immunohistochemistry.
In order to localize cellular expression of PrPC within the tissues analyzed, immunohistochemistry was performed using the anti-PrP SAF-32 monoclonal antibody. Tissues from each of the three animals sampled were analyzed by immunohistochemistry. Results presented are typical for multiple experiments. No staining was observed in the negative control sections incubated with normal horse serum instead of SAF-32 antibody (shown in inserts and some specific sections).
Neural tissues. Among tissues analyzed, the greatest levels and most widely distributed PrPC immunostaining was observed in the nervous system. PrPC labeling in the cerebellum was confined to the gray matter and appeared homogenous and diffuse on neuron bodies and the neuropil (). At the cellular level, immunoreactivity for PrPC was present in unmyelinated fibers, cells of the granular layer (GL), and stellate and basket cells of the molecular layer (ML) (). Purkinje cells observed in all the extensions of the central layer showed intense PrPC staining (). Similarly, immunoreactivity for PrPC was intense in neuronal bodies of the solitary tract nucleus in the obex (). Glia cells, presumably astrocytes and oligodendrocytes observed around neurons showed moderate levels of PrPC labeling (). Immunopositivity in cerebellum and obex was supported by the lack of reactivity in the negative controls ( and inserts). Immunoreactivity for PrPC was analyzed in the thoracic portion (Pars thoracalis) of the spinal cord (). Despite the presence of immunoreactive tracts in the white matter (WM), the majority of the staining was confined to the gray matter (GM). Analysis of PrPC distribution in peripheral nerves was performed in transverse sections obtained from the sciatic nerve (). PrPC labeling was restricted to neural fibers contained in nerve fascicles. No reactivity for PrPC was observed in the connective tissue forming the perineurium (P).
Lymphoreticular tissues. Lobules in the cortex (Cx) of the thymus were intensely labeled for PrPC (). Observation with higher magnification revealed a cell-specific staining associated with thymocytes in the cortical area ( and C). Less intense immunoreactivity for PrPC was detected in epithelial cells located in the medulla (M) (). The intense, widely-distributed immunoreactivity observed in the thymus contrasted with a scattered staining detected in the spleen (). PrPC-positive cells with the appearance of myeloid dendritic cells (DCs) were located in the perilymphoid zones of the red pulp (RP) immediately adjacent to nodules of white pulp (WP). Higher magnification showed cell-specific staining presumably associated to myeloid DCs ( and F). Mesenteric lymph nodes showed cellular PrPC staining located in germinal centers (GC) and lymphocytes coronas (LC) of secondary lymphoid follicles (LF) in the cortical area (). PrPC-positive cells in the lymph node were presumably lymphocytes of the B lineage and follicular DCs ( and I).
Digestive tissues. Immunohistochemical analysis for PrPC was performed in the ileum section of the intestine. Staining was intense and restricted to presumably neural cells present in the lamina propia between intestinal crypts, between the inner and outer layers of the muscularis, and parallel to the inner circular muscular layer ( and B). Clusters of parasympathetic ganglion cells associated with the myenteric plexus showed intense PrPC staining throughout the extension of the ileum sections (). In pancreas, PrPC labeling was restricted to the islets of Langerhans ( and E). No staining was observed in the exocrine pancreatic tissue. Liver tissue showed no detectable immunoreactivity PrPC ().
Striated and cardiac muscle. PrPC immunoreactivity for was not observed in the skeletal muscle sections (). PrPC labeling was detected in small, unidentified structures located adjacent to cardiomyocytes () but no immunolabeling was observed in cardiomyocytes proper.
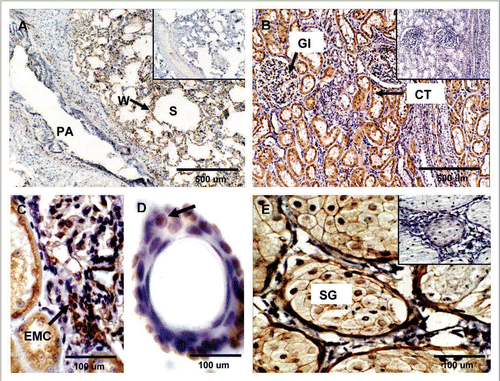
Lung, kidney and skin. PrPC labeling in the lung was mainly associated to the alveolar wall (W) (). In the kidney, PrPC positive staining was observed in proximal convoluted tubules (CT) and medullary collecting ducts (). Renal glomeruli (Gl) showed strong PrPC labeling associated with extraglomerular mesangial cells (EMC) and moderate labeling in podocytes and endothelial cells ( and C). The skin tissue sample displayed PrPC labeling in keratinocytes localized in the epidermis and outer sheaths of the hair follicle (). Furthermore, horizontal sections at the level of the dermis allowed the detection of PrPC staining in sebaceous glands ().
Discussion
We present here a quantitative and qualitative evaluation of PrPC expression in 15 bovine somatic tissues. PrPC expression levels varied widely between tissues and as expected were greater in the CNS and thymus and less in the other tissues examined. The broad tissue distribution of PrPC expression pattern and wide variation in PrPC expression level between tissues, together with its conserved molecular structure suggest an important biological function for this protein. However, apart from its central role in prion disease, the normal function of PrPC is unknown.
Transmission of TSEs, including BSE, can occur through consumption of PrPSc-contaminated feedstuffs.Citation21 Ingested PrPSc enters the host via the alimentary canal where it subsequently induces conversion of host-encoded PrPC to PrPSc. The mechanism of conversion is poorly understood but is thought to require intermolecular contact between infectious PrPSc and host-encoded PrPC.Citation6 Newly-converted, host-derived PrPSc subsequently drives further conversion and replication of endogenous PrPC.Citation7 While initial conversion and replication may occur in alimentary tissues, neuropathogenesis becomes manifest only after a long incubation period (>2 years in BSE), where PrPSc replication reaches the CNS presumably in anterograde fashion through peripheral nerves.Citation7
Evaluation of tissues from TSE-infected animals and humans indicate that the affected tissues correspond to those that express relatively high levels of endogenous PrPC. For example, in BSE-infected cattle PrPSc has been found in myenteric plexi, peripheral neurons, spinal cord and brainstem.Citation22,Citation23 In our quantitative analysis each of these tissues was shown to express relatively high levels of PrPC. On the other hand, skeletal muscle, spleen, mesenteric lymph nodes, and liver from those BSE-infected animals have not been found to contain detectable PrPSc by immunohistochemistry or western blotCitation22 nor have they been shown to be infectious in highly sensitive bioassays in mice.Citation24 Similarly, our quantitative analysis revealed that these tissues express relatively less PrPC compared to neuronal tissues prone to BSE infection. These observations are consistent with the view that a component of prion infection depends on endogenous PrPC expression level.Citation6,Citation14
To protect consumers of US beef from BSE, the United States Department of Agriculture has banned the consumption of tissues known to be infectious for BSE in affected animals.Citation22 These specified risk materials (SRM) include brain, skull, eyes, trigeminal ganglia, spinal cord, central portions of the thoracic, lumbar and sacral vertebrae and dorsal root ganglia of cattle 30 month of age and older, and tonsils and distal intestinal ileum of all cattle.Citation25 Our data covered a subset of these tissues (brain, spinal cord and ileum). PrPC was abundantly expressed in brain and spinal cord. The ileum expressed a somewhat lesser amount than these tissues. Nevertheless, immunohistochemistry revealed intense, localized PrPC staining in myenteric plexi within the ileal wall. The myenteric plexi lie in close proximity to the intestinal lumen and potentially to any ingested PrPSc.Citation23 Ileal myenteric plexi are thus candidate sites for initial conversion of endogenous PrPC to PrPSc during BSE transmission. Overall, SRM tissues correspond to tissues in our study that express relatively high levels of PrPC.
Our immunohistochemical analysis of the obex showed intense PrPC immunoreactivity in the neuropil, neuroglia and neuronal bodies of the solitary tract nucleus. This area of the medulla oblongata is commonly used for BSE diagnosis due to consistent early accumulation of PrPSc.Citation26 Neuronal bodies expressing increased levels of PrPC seem particularly sensitive to PrPSc neurotoxicity, which may explain the intense neuronal degeneration of the obex during PrPSc infection.Citation26,Citation27
Among non-neural tissues examined here, the greatest levels of PrPC expression were detected in the thymus. Our immunohistochemical analysis showed PrPC labeling in the thymic cortex associated with thymocytes. Previous studies in mice have described similar immunodetection in epithelial cells of the mouse thymic medulla and cortex.Citation16 Expression of PrPC is regulated during lymphocyte development in both thymus and bone marrow.Citation28,Citation29 Studies in mice described a rapid accumulation of PrPSc in the thymus after intracerebral scrapie inoculation, suggesting an active role of this organ in replication of PrPSc.Citation30 While we could find no reports in the literature that evaluated the presence of PrPSc or infectivity in the thymus of BSE-infected cattle, the high level of PrPC expression observed here suggests the thymus as a potential candidate for BSE infection.
Despite the reported involvement of the spleen in TSE pathogenesis, this organ expressed relatively small amounts of PrPC in cattle. Most cell types and stromal elements did not stain for PrPC and staining was restricted to a few cells in the marginal zone of the white pulp. Sheep orally inoculated with BSE agent revealed deposition of PrPSc in the same marginal zone of the white pulp where myeloid DCs positive cells were observed in our study.Citation31 Despite these findings, other reports have failed to detect PrPSc deposition in the spleen of BSE-infected cattle.Citation21,Citation22
In the intestine, we detected PrPC immunostaining in neural cells occupying the lamina of enteric crypts and in neurons located within intestinal muscularis as well as in the parasympathetic myenteric plexus. BSE transmission under natural circumstances is widely believed to occur via the oral route.Citation21 Following experimental oral inoculation, PrPSc accumulation is first observed in the gut and in gut-associated lymphoid and neural tissues.Citation21 Thus, initial conversion and amplification of PrPSc likely occurs in PrPC-expressing intestinal tissues. Our results concur with others that bovine intestinal tissues express PrPC,Citation32 and could thus serve as a primary substrate for initial PrPC to PrPSc conversion during infection.Citation33
Our analysis of mesenteric lymph nodes revealed PrPC-positive cells in germinal centers and surrounding areas of the lymphoid follicles. The location of these cells coincides with that of B lymphocytes and follicular DCs.Citation34,Citation35 Both cell types play direct and indirect roles in PrPSc pathogenesis. In mice orally inoculated with PrPSc, follicular DCs are critical for PrPSc replication and accumulation in mouse lymphoid tissues and participate in neuroinvasion.Citation36 PrPC is expressed in bovine follicular DCsCitation37 and could thus in theory play a role in BSE pathogenesis.
Previous studies have reported expression of PrPC in murineCitation16 and bovineCitation38 pancreatic tissue restricted to a subset of cells in the islets of Langerhans, suggesting a role for PrPC in islet function. However, PrPSc has not been detected in the pancreas of BSE-infected cattle.Citation22
Evidence of expression of PrPC in bovine skeletal muscle has important implications for the potential transmission of BSE through the consumption of beef products. Our data showed low (4.2% relative to levels in the cerebellum, p < 0.05) levels of PrPC in bovine skeletal muscle. This reduced level suggests a low propensity for PrPC to PrPSc conversion and PrPSc accumulation even in BSE-infected animals. This observation is consistent with the lack of observed BSE infectivity of skeletal muscle from BSE-infected cattle.Citation21,Citation22
In the kidney PrPC was detected in glomeruli, convoluted tubules and medullary collecting ducts. These observations confirm and extend the results of a previous report that indicated the presence of PrPC in bovine glomeruli.Citation39 Scrapie-infected hamsters and CJD patients have been shown to excrete PrPSc in urine and PrPSc has been detected in the collecting tubules of scrapie-infected hamsters that shed PrPSc in urine.Citation40 While in principle the presence of PrPC in the bovine kidney may render it susceptible to prion infection, PrPSc has not been detected in the urine of BSE-infected cattleCitation21 and to our knowledge, kidneys from BSE-infected cattle have not been evaluated for the presence of PrPSc or BSE infectivity.
Our study showed expression of PrPC in bovine skin where it was observed in epidermal keratinocytes. PrPC was also expressed in sebaceous glands located in horizontal tissue sections of the skin at the level of the dermis and in cells surrounding the inner and the outer root sheet of hair follicles. PrPC has been reported in human skin as well.Citation41 TSE infectivity and PrPSc has been described in the skin of affected rodents and sheep.Citation42 Most PrPSc was associated with neurons innervating the skin and the skin thought to have been infected centrifugally via neurons. To our knowledge skin from BSE-infected cattle has not been evaluated for PrPSc or infectivity.
Our results support previous studies in cattle and other species that reveal PrPC expression in a wide range of somatic tissues. In addition, our study reveals that PrPC expression levels vary over a wide (>10 fold) range in the tissues examined here. The level of PrPC expression in a particular tissue is important as it may determine its potential for prion infection. Moreover, the identity and the extent to which various cells and tissues express PrPC may provide suggestions for further studies into the function of this unusual and enigmatic protein.
Materials and Methods
Tissue collection.
Bovine tissues were obtained from three, 13-month-old healthy Angus steers. Animals were slaughtered at an abattoir in the Department of Food Science and Technology at Virginia Tech. Samples of the following tissues were collected within 20 min of slaughter: cerebellum (hemisphere), obex (solitary tract nucleus), spinal cord (Pars thoracalis), peripheral nerve (sciatic), thymus (intrathoracic), spleen, lymph node (mesenteric), ileum, pancreas, liver (caudate lobe), skeletal muscle (semitendinosus), heart (ventricle), lung (cranial lobule), kidney and skin (flank). Samples for western blot were placed in a glass container and frozen on dry ice. For immunohistochemistry, samples were fixed in 10% formalin.
Western blot.
Tissue samples (<700 mg) were thawed and homogenized (10 w/v) in ice-cold lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton-X-100, 1% deoxycholate, 0.1% SDS) using a pestle homogenizer (Fisher Scientific, Hampton, NH). Homogenates were centrifuged at 13,500 rpm for 5 min and the supernatants recovered. Total protein concentrations were determined in the raw homogenates using a Bicinchoninic acid (BCA) kit (Pierce, Rockford, IL) according to the manufacturer's instructions. Samples were prepared for SDS-PAGE by mixing 1:1 (v/v) with Laemmli buffer (BioRad Laboratories, Hercules, CA) and heated at 98°C for 5 min. Raw homogenate protein concentrations were used to calculate volumes of Laemmli-treated sample containing 20 µg of total protein, which were then loaded onto to each lane and separated by SDS-PAGE in 12% gels (BioRad Laboratories). Electrophoresis was performed under reducing conditions at 125 V for 60 min. Proteins were then transferred onto polyvynilidine fluoride (PVDF) membranes (Immobilon-FL; Millipore Corp., Billarica, MA) by electroblotting at 100 V for 1 h. Membranes were blocked by immersion in blocking buffer (LI-COR Corporation, Lincoln, NE) for 1 h with agitation. Membranes were probed simultaneously for PrPC and a reference housekeeping protein, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). PrPC was probed with a mouse monoclonal antibody directed against amino acid sequence 59–89 located in the N-terminal octapeptide repeat region (SAF-32, 1:400; Cayman Chemical Company, Ann Arbor, MI). GAPDH was probed with a rabbit polyclonal anti-GAPDH antibody (FL-335, 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA). Both primary antibodies were diluted in 0.1% Tween-20 in blocking buffer and incubated at room temperature for 1 h on a rotating platform. Membranes were then washed four times in 0.1% Tween-20 in 0.1 M phosphate-buffered saline at pH 7.4 (PBS) for 5 min each. To detect bound primary antibodies, membranes were incubated with fluorescent-tagged secondary antibodies against mouse and rabbit IgG (LICOR Biotechnology, IRdyes 700 and 800, respectively) diluted 1:5,000 in blocking buffer plus 0.1% Tween-20 for 30 min on a rotating platform. Pixel intensities of PrPC and GAPDH-specific bands were detected and quantified as integrated intensity values using an Odyssey infrared imaging system (LI-COR Biotechnology). Expression of PrPC in each tissue was normalized to GAPDH levels and calculated as a percentage of that in cerebellum, the tissue showing the highest expression level ().
Immunohistochemistry.
Formalin-fixed tissues were embedded in paraffin and sectioned at 5–7 µm using a microtome (Historange, LKB, Bromma, Sweden). Tissue sections were mounted on adhesive slides (Newcomer Supply, Middleton, WI) and incubated overnight at 37°C. Mounted tissues were deparaffinized in xylene and dehydrated in serial alcohol solutions. Slides were subjected to an antigen unmasking protocol by autoclaving at 120°C for 5 min in an unmasking solution (Vector Laboratories, Burlingame, CA). Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide diluted in PBS for 30 min. Tissues were then rinsed two times in PBS and blocked in 2.5% horse serum for 15 min. PrPC was specifically detected by overnight incubation at room temperature with primary antibody SAF-32 (1:400) diluted in 1.5% equine serum solution (Vector Laboratories). After two washes in PBS, bound primary antibody was detected using a horseradish peroxidase-tagged horse anti-mouse secondary antibody (Vector Laboratories) for 10 min at room temperature. Immune complexes were visualized using 3,3′-diaminobenzidine (DAB) substrate for 5 min or until the signal became visible. Probed sections were then counterstained with hematoxilyn and rehydrated in serial alcohol solutions. Sections were mounted with Permount mounting medium (Fisher Scientific) and coverslides. Neighboring sections were used for the following experimental controls: (1) positive control using obex tissue, (2) replacement of the primary antibody with non-immune serum, (3) omission of the secondary antibody, and (4) incubation with diaminobenzidine solution alone. Digital photos of tissue sections were obtained using bright microscopy (Olympus Vanox-T, Tokyo, Japan).
Data analysis.
Tissues for each of the three animals were run in three independent gels and quantified separately. Values of expression of PrPC were transferred to a spreadsheet and then analyzed using SAS software (version 9.3.1, SAS Institute Inc., Cary, NC). Data was normalized to logarithmic scale in base 10 for normality and mean values for each animal were compared by one-way ANOVA. PrPC expression values for individual tissues were compared to the tissue with the highest expression (cerebellum) using Dunnet's t-test. Significant differences (p < 0.05) between tissues were analyzed using Duncan's multiple comparison test.
Abbreviations
| BSE | = | bovine spongiform encephalopathy |
| PrPC | = | cellular prion protein |
| CJD | = | Creutzfeldt-Jakob disease |
| PrPSc | = | scrapie prion protein |
| Prnp | = | prion gene |
| TSE | = | transmissible spongiform encephalopathy |
Figures and Tables
Figure 1 Representative western blot for the expression of PrPC in bovine tissues. (A) PrPC displayed three distinct migration bands corresponding to molecular weights of 35, 28 and 25 kDa. PrPC was detected in all tissues analyzed with greatest expression in CNS tissues (cerebellum, obex, spinal cord). GAPDH was used as control protein (30 kDa) and was present in all samples. (B) Cerebellum, obex and spinal cord showed the highest (p < 0.05) levels of immunoreactivity for PrPC. Thymus expressed highest levels of PrPC among non-neural tissues. Different superscripts letters indicate significant differences (p < 0.05). Ce, cerebellum; Ob, obex; SC, spinal cord; Th, thymus; In, intestine; Ne, nerve; He, heart; Sp, spleen; Lu, lung; Mu, muscle; Ki, kidney; LN, lymph node; Sk, skin; Pa, pancreas; Li, liver.
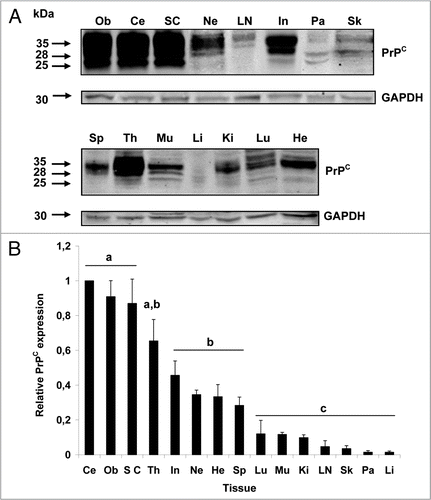
Figure 2 Expression of PrPC in bovine neural tissues. Transverse tissue section incubated with SAF-32 antibody and stained using peroxidase. (A) PrPC staining (brown) is intensely present in Purkinje cells (arrows) and cells of the molecular layer (ML) and granular layer (GL) in the cerebellum. Less immunoreactivity is observed in the white matter (WM). (B) Higher magnification shows intense staining in fibers of the ML, Purkinje cells (arrows) and neurons of the GL. (D) In the solitary tract nucleus of the obex, PrPC is associated to neuronal bodies, neuropil and neuroglia. (E) Higher magnification shows labeling of PrPC in neuronal bodies, appendixes and glial cells (arrow-heads). (G) PrPC immunoreactivity in the spinal cord is confined to the gray matter (GM) with low intensity in the white matter (WM). (H) In the sciatic nerve, PrPC staining is restricted to neural fibers associated in fascicles (F). No PrPC labeling was observed in the perineurium (P). Inserts and figures C (cerebellum) and F (obex) represent serial sections incubated with non-immune horse serum instead of SAF-32 antibody (negative control).
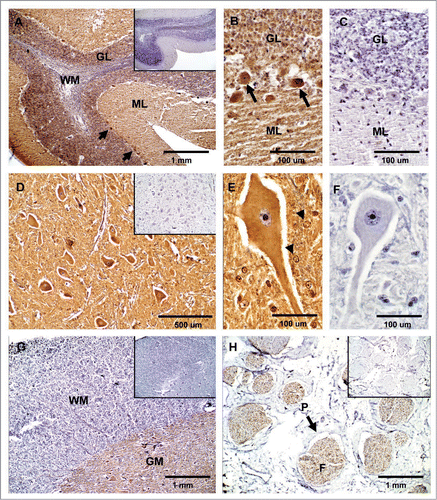
Figure 3 Expression of PrPC in bovine lymphatic tissues. (A) PrPC-specific labeling is greatest in the cortex (Cx) of the thymus and moderate in the medulla (M). (B and C) Higher magnification in the cortex area shows PrPC positive (arrows) and negative (arrow-heads) thymocytes. (D) In the spleen, staining for PrPC was observed in perilymphoid zones surrounding nodules of white pulp (WP) (RP, red pulp). (E and F) Higher magnification shows presumably PrPC positive myeloid DCs (arrow) in the spleen. (G) PrPC-specific labeling is associated with lymphoid follicles (LF) present in the cortex area of the mesenteric lymph node. (H and I) Higher magnification evidence specific staining for PrPC presumably associated with lymphocytes (arrow) surrounding the lymphocyte corona (LC) and germinal centers (GC). Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative controls).
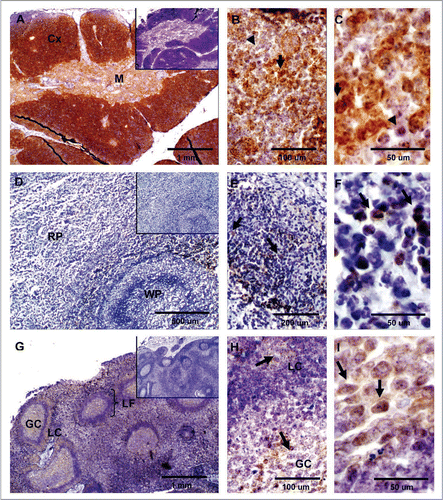
Figure 4 Expression of PrPC in bovine digestive tissues. (A) Sagittal tissue section of the ileum shows PrPC-specific labeling in lamina propia (*), muscularis (arrowhead) and myenteric plexus (arrow). (B) Higher magnification of neurons positive for PrPC in the lamina propia (arrow in B and * in A). (C) PrPC is highly expressed in parasympathetic ganglion cells forming the myenteric plexus (arrow in Fig. A and C). (D) PrPC positive staining is restricted to the endocrine pancreas in the islets of langerhans. (E) Higher magnification shows specific PrPC-positive pancreatic endocrine cells. (F) No PrPC staining was observed in the liver tissue after incubation with SAF-32 antibody. Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative control).
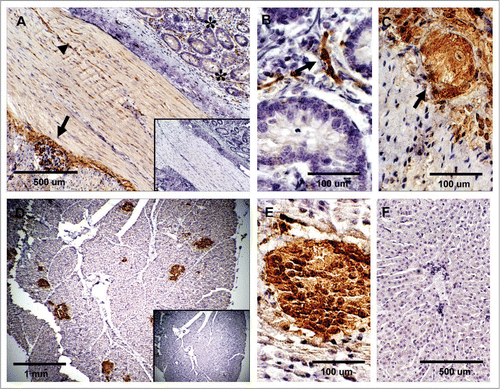
Figure 5 Expression of PrPC in bovine striated and cardiac muscle. (A) No immunoreactivity for PrPC was detected in semitendinosus striated muscle after incubation with SAF-32 antibody. (B) PrPC labeling was observed in unidentified structures outside the cardiac muscle fibers; labeling was not observed in cardiac muscle cells of the myocardium. Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative control).
Figure 6 Expression of PrPC in bovine lung, kidney and skin. (A) PrPC-specific labeling was observed associated with the alveolar wall (W) (arrow) (Alveolar Sacs, S; Pulmonary Artery, PA). (B) In kidney, PrPC immunoreactivity is associated with glomeruli (Gl), proximal convoluted tubules (CT) and collecting ducts in the medulla. (C) Higher magnification of renal glomeruli shows strong PrPC staining in extraglomerular mesangial cells (EMC). (D) PrPC staining in the skin is associated with epidermal cells in hair follicles (arrow). (E) Staining was also present in sebaceous glands (SG) located in the dermis. Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative control).
Acknowledgements
Our sincere thanks to Dr. William Huckle, Dr. Ludeman Eng, Dr. Xiang-Jin Meng, Dr. Jill Sible and Dr. Mary Lynn Johnson for their guidance throughout this study. We would also like to thanks Kathy Lowe and Shireen Hafez for their expert advice on IHC studies. This research was supported by NIH grant R21-NS045908 from the National Institute of Neurological Disease and Stroke. Additional funding was provided by the Virginia-Maryland Regional College of Veterinary Medicine.
References
- McKinley MP, Taraboulos A, Kenaga L, Serban D, Stieber A, DeArmond SJ, et al. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Invest 1991; 65:622 - 630
- Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 1991; 30:7672 - 7680
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA 1993; 90:10962 - 10966
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136 - 144
- Prusiner SB. Prions. Proc Natl Acad Sci USA 1998; 95:13363 - 13383
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, et al. Mice devoid of PrP are resistant to scrapie. Cell 1993; 73:1339 - 1347
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 2001; 24:519 - 550
- Milhavet O, Lehmann S. Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res Rev 2002; 38:328 - 339
- Roucou X, Giannopoulos PN, Zhang Y, Jodoin J, Goodyer CG, LeBlanc A. Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells. Cell Death Differ 2005; 12:783 - 795
- Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, et al. Signal transduction through prion protein. Science 2000; 289:1925 - 1928
- Schneider B, Mutel V, Pietri M, Ermonval M, Mouillet-Richard S, Kellermann O. NADPH oxidase and extracellular regulated kinases ½ are targets of prion protein signaling in neuronal and nonneuronal cells. Proc Natl Acad Sci USA 2003; 100:13326 - 13331
- Steele AD, Emsley JG, Ozdinler PH, Lindquist S, Macklis J. Prion protein (PrPC) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci USA 2006; 103:3416 - 3421
- Bueler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med 1994; 1:19 - 30
- Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 1996; 15:1255 - 1264
- Bendheim PE, Brown HR, Rudelli RD, Scala LJ, Goller NL, Wen GY, et al. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology 1992; 42:149 - 156
- Ford MJ, Burton LJ, Morris RJ, Hall SM. Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 2002; 113:177 - 192
- Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol 1995; 76:2583 - 2587
- Tichopad A, Pfaffl MW, Didier A. Tissue-specific expression pattern of bovine prion gene: quantification using real-time RT-PCR. Mol Cell Probes 2003; 17:5 - 10
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 1997; 389:498 - 501
- Diaz-San Segundo F, Salguero FJ, de Avila A, Espinosa JC, Torres JM, Brun A. Distribution of the cellular prion protein (PrPC) in brains of livestock and domesticated species. Acta Neuropathol 2006; 112:587 - 595
- Espinosa JMM, Castilla JRM, Torres J. Progression of prion infectivity in asymptomatic cattle after oral bovine spongiform encephalopathy challenge. J Gen Virol 2007; 88:1379 - 1383
- Iwata N, Sato Y, Higuchi Y, Nohtomi K, Nagata N, Hasegawa H, et al. Distribution of PrPSc in cattle with bovine spongiform encephalopathy slaughtered at abattoirs in Japan. Jpn J Infect Dis 2006; 59:100 - 107
- Terry LA, Marsh S, Ryder SJ, Hawkins SA, Wells GA, Spencer YI. Detection of disease-specific PRP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet Rec 2003; 152:387 - 392
- Courageot M, Daude N, Nonno R, Paquet S, Di Bari M, Le Dur A, et al. A cell line infectible by prion strains from different species. J Gen Virol 2008; 2008:341 - 347
- USDA, United States Department of Agriculture. Prohibition of the use of specified risk materials for human food and requirements for the disposition of non-ambulatory disabled cattle. Food Safety and Inspection Service. 9 Federal Register 2007; 72:38700 - 38730
- Wells GAH, Hancock RD, Cooley WA, Richards MS. Bovine spongiform encephalopathy: diagnostic significance of vacuolar changes in selected nuclei of the medulla oblongata. Vet Rec 1989; 125:521 - 524
- Ford M, Burton L, Graham C, Frobert Y, Grassi J, Hall S, et al. A marked disparity between the expression of prion protein and its message by neurons of the CNS. Neuroscience 2002; 111:5333 - 5551
- Kubosaki A, Yusa S, Nasu Y, Nishimura T, Nakamura Y, Saeki K, et al. Distribution of cellular isoform of prion protein in T lymphocytes and bone marrow, analyzed by wild-type and prion protein gene-deficient mice. Biochem Biophys Res Commun 2001; 282:103 - 107
- Liu T, Li R, Wong B, Liu D, Pan T, Petersen R, et al. Normal cellular prion protein is preferentially expressed on subpopulations of murine hemopoietic cells. J Immunol 2001; 166:3733 - 3742
- Muramoto T, Kitamoto T, Tateishi J, Goto I. The sequential development of abnormal prion protein accumulation in mice with Creutzfeldt-Jakob disease. Am J Pathol 1992; 140:1411 - 1420
- Andreoletti O, Morel N, Lacroux C, Rouillon V, Barc C, Tabouret G, et al. Bovine spongiform encephalopathy agent in spleen from an ARR/ARR orally exposed sheep. J Gen Virol 2006; 87:1043 - 1046
- Miyazawa K, Kanaya T, Tanaka S, Takakura I, Watanabe K, Ohwada S, et al. Immunohistochemical characterization of cell types expressing the cellular prion protein in the small intestine of cattle and mice. Histochem Cell Biol 2007; 127:291 - 301
- van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Scrapie-associated prion protein in the gastrointestinal tract of sheep with scrapie. J Comp Pathol 1999; 121:55 - 63
- Klein MA, Frigg R, Raeber AJ, Flechsig E, Hegyi I, Zinkernagel RM, et al. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nat Med 1998; 4:1429 - 1433
- Thielen C, Melot F, Jolois O, Leclercq F, Tsunoda R, Frobert Y, et al. Isolation of bovine follicular dendritic cells allows the demonstration of a particular cellular prion protein. Cell Tissue Res 2001; 306:49 - 55
- Mabbot NA, Mackay F, Minns F, Bruce ME. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med 2000; 6:719 - 720
- Terry LA, Marsh S, Ryder SJ, Hawkins SA, Wells GA, Spencer YI. Detection of disease-specific PRP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet Rec 2003; 152:387 - 392
- Amselgruber WM, Buttner M, Schlegel T, Schweiger M, Pfaff E. The normal cellular prion protein (PrPC) is strongly expressed in bovine endocrine pancreas. Histochem Cell Biol 2006; 125:441 - 448
- Amselgruber WM, Steffl M, Didier A, Martbauer E, Pfaff E, Buttner M. Prion protein expression in bovine podocytes and extraglomerular mesangial cells. Cell Tissue Res 2006; 324:497 - 505
- Shaked GM, Shaked Y, Kariv-Inbal Z, Halimi M, Avraham I, Gabizon R. A protease-resistant prion protein isoform is present in urine of animals and humans affected with prion disease. J Biol Chem 2001; 276:31479 - 31482
- Pammer J, Weninger W, Tschachler E. Human keratinocytes express cellular prion-related protein in vitro and during inflammatory skin diseases. Am J Pathol 1998; 153:1353 - 1358
- Thomzig A, Schulz-Schaeffer W, Wrede A, Wemheuer W, Brenig B, Kratzel C, et al. Accumulation of pathological prion protein PrPSc in the skin of animals with experimental and natural scrapie. PLoS Pathog 2007; 3:66