Abstract
Amyloid assemblies are associated with several debilitating human disorders. Understanding the intra- and extracellular assembly of normally soluble proteins and peptides into amyloid aggregates and how they disrupt normal cellular functions is therefore of paramount importance. In a recent report, we demonstrated a striking relationship between reduced fibril length caused by fibril fragmentation and enhanced ability of fibril samples to disrupt membranes and to reduce cell viability. These findings have important implications for our understanding of amyloid disease in that changes in the physical dimensions of fibrils, without parallel changes in their composition or molecular architecture, could be sufficient to alter the biological responses to their presence. These conclusions provide a new hypothesis that the physical dimensions and surface interactions of fibrils play key roles in amyloid disease. Controlling fibril length and stability toward fracturing, and thereby the biological availability of fibril material, may provide a new target for future therapeutic strategies towards combating amyloid disease.
Background to Amyloid Assembly and Cytotoxicity
The assembly of normally soluble proteins and peptides into fibrillar amyloid deposits and the cytotoxicity coupled with amyloid aggregation are associated with numerous devastating human disorders, including type II diabetes mellitus and Alzheimer, Parkinson and Creutzfeldt-Jakob diseases.Citation1 The assembly of the fibrillar aggregates, characteristic of amyloid disease, is believed to occur through nucleated polymerisation and fibril elongation that involves the addition of assembly-competent precursors to fibrillar ends.Citation2 Different oligomeric species that accumulate during the early stages of fibril assembly have been suggested as the origin of the cytotoxicity associated with amyloid disease, and fibril fragmentation that shortens fibril length and increases the number of fibrils is a key secondary process that accelerates amyloid assembly.Citation2,Citation3 Amyloid fibrils are thought to be mechanically and biologically stable assemblies.Citation4 They have a characteristic cross-β core-architecture,Citation5 usually appear as long-straight and unbranched structures with width in the order of ∼10 nm and lengths up to several micrometres,Citation4,Citation6,Citation7 and can be formed from potentially all proteins and peptides irrespective of their amino acid sequence.Citation8 Despite being formed by physiologically available proteins or peptides in situ, amyloid fibrils can be regarded as stable, persistent, nano-scale particles that can produce serious threats to cellular integrity and function when produced in an uncontrolled manner in vivo, similar to other man-made or naturally occurring nano-scale materials whose properties depend on their size.Citation9 The physical attributes of amyloid fibrils such as length, width and surface area may therefore play important roles in determining how these assemblies affect biological processes and elicit cellular responses.
In recent years, pre-fibrillar oligomers have been implicated as the primary cytotoxic species associated with amyloid formation.Citation10–Citation13 Efforts to identify the species involved in mediating the cytotoxicity associated with amyloid disorders resulted, however, in varied reports that have implicated both the soluble pre-fibrillar oligomers and the fibrillar products of assemblyCitation14–Citation16 as the primary cytotoxic species. Taken as a whole, these reports raise the possibility that the determinants of cytotoxicity may not always be associated with a single type of species. These reports also suggest that fibrils themselves or fibril-associated species (i.e., species dynamically associated with fibrils through direct exchange) may also possess cytotoxic potential, and fibrils should not be dismissed as merely the inert products of amyloid assembly. The physical attributes of fibrillar aggregates that influence surface interactions and biological availability may therefore play an important role in disease. Such properties could contribute to the variety of different responses of cells to the presence of amyloid deposits.
Relationship between Fibril Length and Fibril-Associated Cytotoxicity
To investigate the impact of fibrils on the cellular responses to amyloid, in particular how the responses are related to the physical dimensions of the fibrils, we characterised the length distribution of fibrils formed in vitro from β2-microglobulin (β2m) in detail by tapping-mode atomic force microscopy (TM-AFM) single particle image analysis.Citation17,Citation18 In parallel, we assessed the ability of these fibril samples to seed the growth of new fibrils, to disrupt liposome membranes and to alter cell viability.Citation17 Through the application of carefully controlled fragmentation of preformed fibrils of β2m by mechanical agitation using a custom made stirrer equipped with a revolution-counter on the rotor-axis, we generated fibril samples containing an identical monomer equivalent concentration that had indistinguishable molecular architecture,Citation17 but distinct length distributions (, upper plot). When fibril growth of fresh monomer solutions of β2m was seeded by each of these samples, the initial rate of fibril growth was shown to be enhanced for fibril samples containing short fibrils compared with their longer counterparts (, lower plot). The increase in the seeding efficiency as fragmentation proceeds is well described by a model where the initial extension rate is directly proportional to the relative increase in the number of fibril particles as fibrils fragment (), as expected for an assembly mechanism involving monomer addition to fibrillar ends.Citation2 Importantly however, when added to liposomes formed from 80% (w/w) phosphatidyl choline and 20% (w/w) phosphatidyl glycerol within which 50 mM carboxyfluorescein had been encapsulated, the fibril samples were found to be able to cause membrane disruption, resulting in the release of the encapsulated dye. Most remarkably, however, the samples containing shorter fibrils were found to disrupt the liposome membranes more efficiently than their longer counterparts (, center plot). The efficiency of dye release caused by these samples had a complex relationship with fibril length that is not adequately described solely by the increase in the number of fibrils as fragmentation proceeds. This contrasts markedly with the simple relationship between the number of fibril particles and their seeding efficiency (, lower plot), suggesting that the surface area of ends (proportional to the number of fibrils) as well as surfaces along the fibril axis together affect fibril-membrane interactions. The ability of fibril samples to cause a reduction in cell viability was also assayed using 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assays. The results of the MTT assays showed that samples containing shorter fibrils reduced cell viability of SH-SY5Y and RAW 264.7 cells to a greater extent than samples containing longer fibrils (). The same length-dependent effect on liposome membrane integrity and on cell viability assayed by MTT was also observed for fibrils formed from lysozyme or α-synuclein (). The results therefore demonstrate the striking finding that shorter fibrillar samples show an enhanced cytotoxic potential compared with their longer counterparts despite possessing indistinguishable molecular architecture. Fibril length thus inversely correlates with the ability to disrupt membranes and to reduce cell viability. These findings bring fragmentation, and the physical dimensions and surface interactions of amyloid fibrils, into focus as potential key properties affecting the behaviors of amyloid fibrils in disease.
Fibril Fragmentation and Length Dependent Properties in Amyloid Assembly and Cytotoxicity
The finding that fibril samples are capable of disrupting model liposome membranes shown in our studyCitation17 is consistent with the hypothesis that fibril-associated cytotoxicity may result from the damage of cellular membranes, a phenomenon suggested in previous studies to account for the cytotoxicity associated with pre-fibrillar oligomeric species.Citation19 The molecular mechanism of damage remains unclear, and it is possible that fibrils and oligomers act by similar or distinct mechanisms. Several possiblities exist, including the creation of pores, through membrane thinning or via the lowering of the dielectric barrier.Citation19 The plasma membrane could be a major target for the interaction of fibrils with cells, as it is accessible to extracellular fibrils as well as those formed within the cytoplasm of cells. However, deleterious cellular responses may result from the interaction between fibrils and lipid membranes at other sites. Indeed, some fibrils are formed intracellularly, whereas other extracellularly formed fibrils, if a suitable size, may be internalised by cells.Citation20,Citation21 Disruption to intracellular membranes, predominantly by fragmented fibrils, could therefore provide a plausible mechanism for fibril-associated-cytotoxcity.
Fibril fragmentation has recently been implicated as a key process involved in determining the kinetics of amyloid assembly.Citation2,Citation3 Under conditions in which nucleation is slow, as in the case of amyloid formation in disease, the rate of fibril growth can be drastically accelerated by the process of fragmentation through the resulting creation of extension-competent surfaces when fibrils break. Fragmentation of fibril particles has also been shown to affect the phenotype strength of different yeast prion strains, as increased brittleness of fibrils increases the efficiency of prion infection.Citation3 The increase in both the rate of amyloid fibril assembly and the phenotype strength of prions formed from fibrils that are more readily fragmented is a direct consequence of the division of fibril particles caused by fragmentation, which results in an increased number of extension-competent sites. Notably however, fibril fragmentation also reduces fibril length, leading to a reduction of the surface area along the fibril axis per particle. This size-altering effect of fragmentation modifies the ability of fibrils to interact with surfaces, including biological membranes or other fibril particles, even in the absence of changes in the composition or the molecular architecture of fibril material. The combined effects of decreasing the length of individual fibrils and increasing the number of fibrillar particles caused by fibril fragmentation may together contribute to enhance the cytotoxic potential of fibrillar material.
As fibril fragmentation proceeds for both intracellular and extracellular amyloid fibrils, the increase in the number of fibril particles and the decrease in their dimensions result in a number of different effects. Fragmentation increases the number of extension-competent surfaces at fibril ends, leading to an increase in the fibril extension rate.Citation2 The increase in the number of fibrils, in parallel, will raise the dissociation frequency of soluble species, possibly possessing cytotoxic potential, in direct dynamic exchange with fibril ends.Citation22 Reduction in the physical dimensions of fibrils following fragmentation could also result in enhanced fibril-membrane surface interactions and/or reduced fibril-fibril interactions that may increase the biological availability and overall surface activity of fibril deposits. Reducing the overall fibril size could also lead to increased internalisation by cellsCitation20 or increased membrane penetration of extracellular fibrils, giving them an access to potentially more vulnerable internal membranes. The diffusion rates of shorter fibril particles are also enhanced compared with their longer counterparts, which could further increase their biological activity. In all of these scenarios, a decrease in the physical dimensions of fibrils would lead to an enhancement of the capacity to increase fibril load, as well as to an enhancement of the cytotoxic potential of fibril material ().
Implications of Fibril Fragmentation, Physical Dimensions and Surface Interactions for Amyloid Disease
The results of our study on the biological consequence of changes in fibril length caused by fragmentation have several important implications for amyloid disease. Firstly, fibril fragmentation provides a mechanism by which fibril load can be rapidly increased and fibrillar species of reduced dimensions with enhanced cytotoxicity are created. This then leads to the hypothesis that fibril fragmentation may be a key process in amyloidosis, hastening the progression of amyloid disease and enhancing the deleterious cellular response to the presence of amyloid fibrils. Environmental factors in vivo that affect fibril fragmentation may therefore modulate the rate of development of amyloid disease, even for fibrils of the same composition and architecture. Such fragmentation processes in vivo could be caused by direct mechanical stress, thermal motion, or the activity of chaperones such as Hsp104, which has a known ability to fragment fibril samples.Citation23 On the other hand, molecular changes such as alteration of the amino acid sequence of amyloid precursors as a result of genetic mutations may alter the mechanical properties of fibrils and/or their stability towardfragmentation. This could help to explain the different rates of onset of amyloid disease for different protein sequences. In order to further the understanding of amyloid disease, precise information regarding the environmental factors that effect the mechanical properties of amyloid fibrils,Citation4 as well as the molecular determinants of fibril structure, stability and dynamics,Citation6,Citation7,Citation24 and how these properties alter the fragmentation rate, are therefore critical. The precise rates of fibril fragmentation for different amyloid systems or different amyloidogenic sequences represent the key parameter that must be characterised in this case. The rates of fragmentation (the division/replication of fibril particles), with the rates of nucleation (the creation/infection of new fibril particles), and extension (the growth of existing fibril particles), represent the key triad of processes that together determines the size distributions of amyloid species, and may dictate the detailed progress of amyloid disease similar to analogous processes involving microbial and viral infectious and/or pathogenic particles.
Secondly, since fibril length is a crucial parameter in determining the type and the extent of cellular responses to the presence of amyloid, characterisation of the length distribution of fibrils becomes crucial to studies of amyloid disease. Information regarding fibril length will also be vital for characterisation of the process of fibril fragmentation, as fragmentation rates are themselves fibril length dependent.Citation25 While length (but not height/width) was varied through controlled fragmentation in the case of β2m in our recent report,Citation17 other physical dimensions may be significantly perturbed as well in vivo. Thus, methods capable of characterizing the size distributions of amyloid particles in general, such as light scattering,Citation26 sedimentation,Citation27 fluorescence (correlation spectroscopy, or total internal reflection microscopyCitation28), nuclear magnetic resonance (diffusion ordered spectroscopy, NMR-DOSY)Citation24,Citation29 and imaging (electron microscopy or TM-AFMCitation18), are paramount for the study of amyloid disease. In particular, direct imaging methods such as TM-AFM may prove to be particularly useful, at least for studies in vitro, as detailed model free distribution data can be obtained through single particle detection and measurements.Citation18
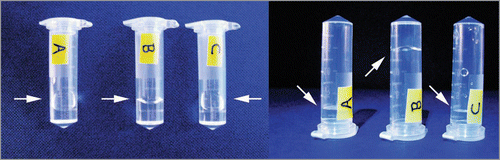
Thirdly, it is evident from our study that not only do different types of aggregation species give rise to different biological responses, but changes in the physical dimensions of aggregates alone, without parallel changes in their composition or molecular architecture, may be sufficient to alter the biological responses to amyloid. Based on our experimental results, the biological responses that are dependent on the dimensions of amyloid aggregates are likely mediated by surface interactions, for example between fibrils and membranes and/or between fibrils themselves. Surface interactions involving nano-particles are known to cause the adsorption of proteins on to particle surfaces depending on the characteristics, physical geometry and as the area to mass ratio of the surfaces presented by the nano-particles.Citation30–Citation32 Nano-scale particles of amyloid species may behave in an analogous manner, as the surfaces presented by amyloid aggregates may be entirely different than their precursor proteins or peptides, and therefore capable of eliciting new biological responses. Given the rigidity of fibrils (which have a persistence length of at least micrometersCitation33), long fibrils (∼micrometers in length) could interact with membranes either through their end surfaces or through their surface along the fibril axis. On the other hand, both types of surfaces may be able to interact concomitantly with membranes for fibrils that are shorter than the size of cells or cellular compartments (in the order of hundred nanometres or shorter), possibly giving rise to enhanced interactions. Thus, for fibrils less than 100 nm in length that are extensively populated in fragmented samples, their larger end surface area per weight material and smaller area per fibril parallel to the fibril axis may result in particles with physical dimensions more optimal for disrupting membranes. In addition, due to the length dependence of fibril-fibril interactions, long fibrils are likely to be more prone to entangle and form clusters or networks that are less biologically active than their unclustered counterparts. For example, the β2m fibril samples used in our study containing, on average, 1.4 µm fibrils17 display gel-like properties (, sample B). By contrast, the same sample fragmented to an average length of ∼400 nm (, sample C) displays properties similar to a dilute aqueous solution (, sample A). The type, strength and balance of surface interactions between amyloid species, as well as between amyloid species and membranes, proteins or other macromolecules may therefore be the crucial determinates of cellular responses to the presence of amyloid fibrils.
Implications for Therapeutic Approaches to Amyloid Disease
Our study has indicated that both the time course of amyloidogenesis and the cellular responses to the presence of amyloid fibrils depends on the extent of fibril fragmentation. The length distribution and the surface properties of amyloid fibrils could thus be key parameters in amyloid disease. These conclusions suggest that the hallmarks of amyloid disease do not only simply depend on the quantity of amyloid deposits, or the nature of amyloid precursors, but also on the biological availability of amyloid aggregates and their surfaces properties. Thus, therapeutic strategies that reduce the amount of aberrant surfaces presented by amyloid fibrils, or which reduce the biological availability of amyloid fibrils in general could be successful in limiting the growth rate of amyloid deposits as well as reducing the cytotoxic effects of amyloid itself. For example, strategies to stabilise fibrils against fracturing, to induce clumping of fibril fragments, and/or to coat the surfaces of amyloid fibrils or fibrillar deposits could all lead to reduction of the deleterious biological activities associated with the presence of amyloid. These strategies may prove to be powerful new directions for future therapies to combat the devastating consequences of amyloid disease.
Abbreviations
| β2m | = | β2-microglobulin |
| MTT | = | 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide |
| TM-AFM | = | tapping-mode atomic force microscopy |
Figures and Tables
Figure 1 The effect of fibril fragmentation on length, seeding capability, capacity to cause membrane disruption and cytotoxic potential of fibril samples. (A) Change in weight average fibril length as measured using TM-AFM single particle image analysisCitation18 (upper), ability to disrupt a model lipid membrane formed from 80% (w/w) phosphatidyl choline and 20% (w/w) phosphatidyl glycerol (middle), and initial fibril extension rate when added to fresh monomer solutions (lower) as function of the length of time a preformed β2m fibril sample was fragmented by stirring at 1,000 r.p.m. For the initial fibril extension rate (lower) and dye release efficiency (middle), calculated curves based on a model where the observed changes are directly proportional to the relative increase in the number of fibril particles as fibrils fragment (dashed lines), or best fit non-linear power-law relationship (solid lines) are also shown in each case. The difference in the two curves in the case of dye release efficiency illustrates that membrane disruption follows a more complex relationship with fibril length than fibril extension rate, in a manner that cannot be described solely by the increase in the number of fibrils as fragmentation proceeds. (B) Ability to disrupt liposome membranes (dye release assay), and to decrease cell viability (MTT assay) by samples containing short (white bars) or long (grey bars) fibrils of lysozyme (upper plot), α-synuclein (center plot) or β2m (lower plot). Relative dye release efficiency and relative cell viability were normalised against the effect of long fibrils for comparison. The error bars represent one standard error. Negative stain transmission electron micrographs of the fibril samples (top: lysozyme, centre: α-synuclein, bottom: β2m) are shown to the right, with scale bars representing 200 nm. In each case, the fragmented sample is shown below its unagitated counterpart. (Data were originally published in The Journal of Biological Chemistry, Xue et al. Fibril fragmentation enhances amyloid cytotoxicity. J Biol Chem 2009; 284:34272–82.Citation17 Copyright, the American Society for Biochemistry and Molecular Biology).
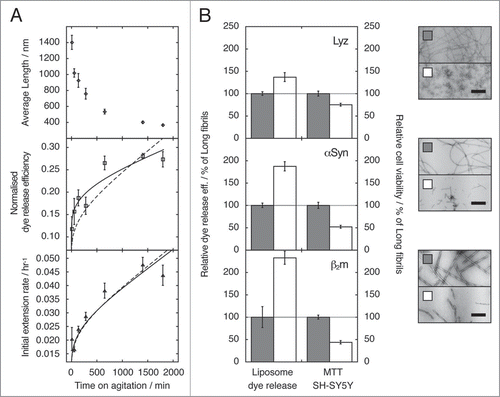
Figure 2 Schematic illustration of the landscape of fibril assembly and fragmentation in relation to the mechanism of fibril associated cytotoxicity. An assembly landscape is illustrated by fibril load plotted against fibril length (upper). The intensity of the red background colour represents the cytotoxic potential. The thick red arrow (I) in the landscape illustrates a representative fibril assembly pathway that would occur in the presence of fibril fragmentation or where nucleation is rapid relative to elongation, resulting in a rapid formation of fibrils with short length distributions. The presence of these short fibrils could lead to enhanced cytotoxicity through decreased fibril-fibril interactions (1) and/or increased fibril-membrane interaction (2). The increased interaction between short fibrils and membrane surfaces could result in membrane damage and a cytotoxic response by fibrils penetrating the membrane (3), growing on the membrane surface (4) or releasing cytotoxic species (5). The thin blue arrow in the assembly landscape (II) illustrates a representative fibril assembly pathway in which little fibril fragmentation occurs or where nucleation is slow relative to elongation, which results in a slow increase of fibril load and the formation of long fibrils. These long fibrils are likely to be less biologically available through increased fibril-fibril interactions (6), decreased interaction with membranes (7) and/or decreased ability of fibrils to pass through the membrane or be taken up by a cell (8) compared with their short counterparts. Fragmentation post-assembly (either mechanical or via chaperones) shortens the average fibril length and thereby enhances the cytotoxic potential without changes to fibril structure (blue to red horizontal arrows III). (Figure was originally published in The Journal of Biological Chemistry, Xue et al. Fibril fragmentation enhances amyloid cytotoxicity. J Biol Chem 2009; 284:34272–82.Citation17 Copyright, the American Society for Biochemistry and Molecular Biology).
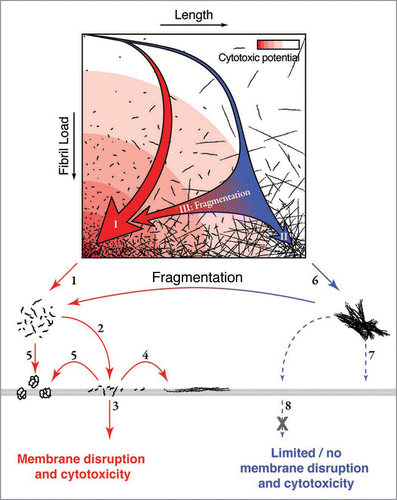
Figure 3 Photographs of a tube with sample solution containing (A) water, (B) unagitated long-straight β2m fibrils displaying gel-like properties and (C) long-straight β2m fibrils that have been fragmented to an average length of ∼400 nm through mechanical stirring. Sample tubes are shown lying down (left photo) or standing in an up side down position (right photo). An arrow indicates the position of the meniscus in each case. The unagitated fibril sample (B) is demonstrating gel-like properties as the meniscus does not follow the tilt of the sample tube (left), nor does the solution readily flow compared with water or fragmented fibril sample (right) likely due to increased viscosity.
Acknowledgements
We thank Walraj Gosal and Steve Homans for all their inputs and comments into this work, and for the co-authorship of many manuscripts, including the Journal of Biological Chemistry paper on which this commentary is based. This study was funded by the Wellcome Trust (grant no 075675) and BBSRC (grant no BB/526502/1).
References
- Chiti F, Dobson CM. Protein misfolding, functional amyloid and human disease. Annu Rev Biochem 2006; 75:333 - 366
- Xue WF, Homans SW, Radford SE. Systematic analysis of nucleation-dependent polymerization reveals new insights into the mechanism of amyloid self-assembly. Proc Natl Acad Sci USA 2008; 105:8926 - 8931
- Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature 2006; 442:585 - 589
- Knowles TP, Fitzpatrick AW, Meehan S, Mott HR, Vendruscolo M, Dobson CM, Welland ME. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 2007; 318:1900 - 1903
- Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, Blake CC. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol 1997; 273:729 - 739
- Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fandrich M. Abeta(1–40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol 2009; 386:869 - 877
- White HE, Hodgkinson JL, Jahn TR, Cohen-Krausz S, Gosal WS, Muller S, et al. Globular tetramers of beta(2)-microglobulin assemble into elaborate amyloid fibrils. J Mol Biol 2009; 389:48 - 57
- Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, Dobson CM. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci USA 1999; 96:3590 - 3594
- Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol 2003; 21:1166 - 1170
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003; 300:486 - 489
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006; 440:352 - 357
- Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc Natl Acad Sci USA 2004; 101:2817 - 2822
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 2008; 14:837 - 842
- Gharibyan AL, Zamotin V, Yanamandra K, Moskaleva OS, Margulis BA, Kostanyan IA, Morozova-Roche LA. Lysozyme amyloid oligomers and fibrils induce cellular death via different apoptotic/necrotic pathways. J Mol Biol 2007; 365:1337 - 1349
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008; 451:720 - 724
- Pieri L, Bucciantini M, Nosi D, Formigli L, Savistchenko J, Melki R, Stefani M. The yeast prion ure2p native-like assemblies are toxic to mammalian cells regardless of their aggregation state. J Biol Chem 2006; 281:15337 - 15344
- Xue WF, Hellewell AL, Gosal WS, Homans SW, Hewitt EW, Radford SE. Fibril fragmentation enhances amyloid cytotoxicity. J Biol Chem 2009; 284:34272 - 34282
- Xue WF, Homans SW, Radford SE. Amyloid fibril length distribution quantified by atomic force microscopy single-particle image analysis. Protein Eng Des Sel 2009; 22:489 - 496
- Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging 2006; 27:570 - 575
- Morten IJ, Gosal WS, Radford SE, Hewitt EW. Investigation into the role of macrophages in the formation and degradation of beta2-microglobulin amyloid fibrils. J Biol Chem 2007; 282:29691 - 29700
- Porter AE, Knowles TP, Muller K, Meehan S, McGuire E, Skepper J, et al. Imaging amyloid fibrils within cells using a Se-labelling strategy. J Mol Biol 2009; 392:868 - 871
- Carulla N, Caddy GL, Hall DR, Zurdo J, Gairi M, Feliz M, et al. Molecular recycling within amyloid fibrils. Nature 2005; 436:554 - 558
- Shorter J, Lindquist S. Hsp104 catalyzes formation and elimination of self-replicating sup35 prion conformers. Science 2004; 304:1793 - 1797
- Platt GW, Xue WF, Homans SW, Radford SE. Probing dynamics within amyloid fibrils using a novel capping method. Angew Chem Int Ed 2009; 48:5705 - 5707
- Hill TL. Length dependence of rate constants for end-to-end association and dissociation of equilibrium linear aggregates. Biophys J 1983; 44:285 - 288
- Lomakin A, Benedek GB, Teplow DB. Monitoring protein assembly using quasielastic light scattering spectroscopy. Methods Enzymol 1999; 309:429 - 459
- Mok YF, Howlett GJ. Sedimentation velocity analysis of amyloid oligomers and fibrils. Methods Enzymol 2006; 413:199 - 217
- Ban T, Goto Y. Direct observation of amyloid growth monitored by total internal reflection fluorescence microscopy. Methods Enzymol 2006; 413:91 - 102
- Baldwin AJ, Anthony-Cahill SJ, Knowles TP, Lippens G, Christodoulou J, Barker PD, Dobson CM. Measurement of amyloid fibril length distributions by inclusion of rotational motion in solution NMR diffusion measurements. Angew Chem Int Ed Engl 2008; 47:3385 - 7
- Linse S, Cabaleiro-Lago C, Xue WF, Lynch I, Lindman S, Thulin E, et al. Nucleation of protein fibrillation by nanoparticles. Proc Natl Acad Sci USA 2007; 104:8691 - 8696
- Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA 2007; 104:2050 - 2055
- Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, Lindman S, Minogue AM, Thulin E, et al. Inhibition of amyloid beta protein fibrillation by polymeric nanoparticles. J Am Chem Soc 2008; 130:15437 - 15443
- Smith JF, Knowles TP, Dobson CM, Macphee CE, Welland ME. Characterization of the nanoscale properties of individual amyloid fibrils. Proc Natl Acad Sci USA 2006; 103:15806 - 15811