Abstract
Transmissible Spongiform Encephalopathies (TSE) or prion diseases are a threat to food safety and to human and animal health. The molecular mechanisms responsible for prion diseases share similarities with a wider group of neurodegenerative disorders including Alzheimer disease and Parkinson disease and the central pathological event is a disturbance of protein folding of a normal cellular protein that is eventually accompanied by neuronal cell death and the death of the host. Prion protein (PrP) is a constituent of most normal mammalian cells and its presence is essential in the pathogenesis of TSE. However, the function of this normal cellular protein remains unclear. The prevention of PRNP gene expression in mammalian species has been undramatic, implying a functional redundancy. Yet PrP is conserved from mammals to fish. Recent studies of PrP in zebrafish have yielded novel findings showing that PrP has essential roles in early embryonic development. The amenability of zebrafish to global technologies has generated data indicating the existence of “anchorless” splice variants of PrP in the early embryo. This paper will discuss the possibility that the experimentalist’s view of PrP functions might be clearer at a greater phylogenetic distance.
Prion Biology and Disease
The prion theory proposes that TSEs are caused by a proteinaceous infectious agent called a prion.Citation1 The endogenously coded prion protein is found in most cells and this normal protein can form a pathological variant PrPSc, that aggregates to form insoluble fibrils. The pathological form of the protein mediates disease by acting as a template for the conversion of its physiological form into copies of itself. The proteinase-resistant form of PrP serves as a marker of infectivity and is used both for diagnostic purposes and as a “tracking device” to investigate pathways followed by the TSE infectious agent as it spreads within the body. However, despite intense research the only general agreement concerning the molecular pathogenesis of prion diseases is that an interference with the normal metabolism of PrP occurs. Thus, further studies are needed on both the molecular pathology and biological function of PrP. The nomenclature for prion protein variants and the corresponding genes can be confusingCitation2 and in this paper we have chosen standard mammalian prion gene and protein nomenclature for mammals (PRNP to denote the gene and PrP to denote the protein). For simplicity we here use PrP and PrP to denote the zebrafish genes and proteins, respectively.
The misfolding of host-encoded PrP and neuroinvasion of the peripheral nervous system are fundamental events in the pathogenesis of prion diseases. The expression of PRNP by host cells is necessary for prion replication and neuroinvasion and the biochemistry of PrP as a glycosylated glycosylphosphatidylinositol (GPI)-anchored membrane protein is broadly established.Citation3 Despite these advances in understanding, numerous issues remain in relating prion biology to prion disease. These issues include the cellular distribution and polarized sorting of PrP and further, the revelance of proteolytic processing of the protein, which separates the flexible amino-terminal tail from the globular domain of the protein. Indeed, no definitive binding partner for PrP has been identified and a multitude of candidate molecules has been suggested including the GPI-anchored proteoglycan Glypican-1, the laminin receptor precursor (LRP) and the cell adhesion proteoglycan N-CAM.Citation4 Yet the most elusive issue for the experimentalist has been the function of PrP. This normal cellular protein has been conserved across the animal kingdom from mammals to fish and appears to be involved in many fundamental cellular processes but also appears not to be essential in mammals.
Molecular Biology and the Arrival of the “-omics”
The function of PrP has been addressed in PRNP gene knockout (KO) models of mouse,Citation5,Citation6 cattleCitation7 and goat,Citation8 but these studies have demonstrated that no dramatic phenotypes affecting development or organogenesis correlate with PRNP null mutations in the mammalian species studied.
The creation of PRNP transgenic mice has been used to study the pathogenesis of scrapieCitation9 and ovine PRNP transgenic cell lines have contributed to the understanding of PrP sub-cellular metabolism.Citation10 A hindrance to the study of prion disease has been the difficulty of transmitting prions to cultured cells, although the description of transmission of prions to an ovine PRNP transgenic rabbit epithelial cell line has demonstrated that cultivated cells from non-neuronal origin can efficiently replicate prions.Citation11 While the power of mouse transgenics in prion research is widely recognized, progress in unraveling the normal function of PrP and the underlying molecular mechanisms of TSE pathogenesis has been slow.
For any cell type, tissue or organism, the full complement of transcribed RNAs and relevant proteins at a given point in time is known as the transcriptome and proteome, respectively. These molecular populations change during development and in response to environmental factors including disease. Microarray technologies for the global investigation of the transcriptome have been used to investigate prion disease. The differential gene expression in prion-infected neuronal cellsCitation12 or brain tissueCitation13–Citation15 from mice, or following induced expression in cell lines that normally do not express PrPCitation16 has been studied. These studies have identified changes in many genes that have implicated inflammatory and stress responses, and shown similarities to other neurodegenerative disorders and alterations in calcium homeostasis. The changes in gene expression varied between studies with the stage of disease and with the cell type examined. Detected changes in gene expression would also be expected to vary with the species examined. The investigation of the transcriptomes of scrapie infected Peyer's patches of sheepCitation17,Citation18 and global approaches to caudal medulla tissues from cattle infected with bovine spongiform encephalopathyCitation19 have shown that 100–200 genes are significantly up-or downregulated. These results revealed that prion infection may cause dysfunction of several molecular networks, including extracellular matrix, cell adhesion, neuroactive ligand-receptor interaction, complement and coagulation cascades, MAPK signaling, neurodegenerative disorder, SNARE interactions in vesicular transport, and the transforming growth factor beta signaling pathways.Citation19 A comparative transcriptomic analysis of the brain of wild-type mice with that of transgenic mice invalidated at PRNP locus either at the zygotic (gene KO) or at adult stages (Cre-lox inducible) demonstrated an unexpectedly low degree of brain transcriptomic modification.Citation20 This lack of response in gene expression is consistent with the observation that the ablation of PRNP gene (PrP null) results in only minor effects on phenotypic expression in the mammals studied.Citation5–Citation8 It has been suggested that important steps in understanding prion disease may be achieved if prion signaling systems were introduced to simpler, genetically tractable organisms such as worms, flies or fish.Citation21 The investigation of prion biology in the zebrafish model has supported this notion. The disturbance of PrP function in zebrafish embryos has correlated with drastic phenotypes and revealed more pronounced effects at the gene expression levels.Citation2,Citation22
Prion Protein Functions and Early Embryonic Development in Zebrafish
The zebrafish is a model species in functional genomicsCitation23–Citation25 and among its attributes has been a genome containing 24,000 genes that have human counterparts (Zv9; www.ensemble.org/Danio rerio/info). As with mouse models, a broad spectre of advanced “-omics” related methods have been established (see zfin.org), including zinc finger nucleases for targeted mutagenesisCitation26 (www.zincfingers.org). The zebrafish has a transparent embryo and adult transparent mutants have been identified. Accordingly zebrafish are widely used for mapping gene expression patterns using fluorescent protein reporter constructs. The genetic tractability of zebrafish has been exploited to study of neurodegenerative diseases such as Alzheimer diseaseCitation27 and Parkinson disease.Citation28
The PRNP gene is phylogenetically conserved and in zebrafish, two transcripts and the corresponding genes encoding prion proteins, PrP-1 and PrP-2, related to human PRNP have been characterized and a third more divergent prion-related gene, PrP-3, has been described.Citation29 It has been demonstrated that PrP-related proteins from zebrafish are glycosylated and contain a glycosylphosphatidylinositol anchor.Citation30
In zebrafish, onset of endogenous PrP expression occurs within one day post fertilization (dpf).Citation2,Citation29 From recent RNA-seq (RNA deep sequencing) derived early zebrafish development transcriptomes, it can be registered that PrP-2 is present as maternal RNA and persists until early gastrula (50% gastrula epiboly at 5.3 hours post fertilization hpf), while PrP-1 is not present during this very early stage of development.Citation31 Morpholino knock-down experiments have demonstrated that the fish prion genes are essential for embryo survival, which is contrary to findings in knockout mice.Citation2,Citation22
Knockdown of PrP-1 prevented embryos from carrying out gastrulation and led to early developmental arrest.Citation22 The cellular and molecular characterization of the PrP-1 phenotype showed that the gastrulation arrest was preceded by a marked decrease in tissue integrity due to the progressive loss of cell-cell adhesion.Citation22 Knockdown of PrP-2 revealed impaired brain and neuronal development at 24 hpf.Citation2 The observation that PrP-1 gene knockdown can be rescued by both zebrafish PrP-2 and mouse PRNP mRNAs suggests that both zebrafish genes share partial functionality with the mammalian prion gene.Citation22,Citation32,Citation33
Microarray analysis of the PrP-2 knockdown samples using a 16 k oligonucleotide library revealed that 249 genes were significantly differentially expressed. Ingenuity pathway analysis (IPA) identified and mapped 120 of the 249 genes as being genes with mammalian ortholog identifiers, of which 69 were upregulated and 51 downregulated.Citation2 Real-time PCR studies validated the effect on selected candidate genes. This gene expression study was based on depleting the mammalian prion protein ortholog from the developing zebrafish embryo and has linked the zebrafish PrP-2 gene to biological processes including neurogenesis.Citation2 ( and ).
Conclusions and Perspectives on the Future
The conclusion to be drawn from the studies of PrP function in early embryo development is that the disturbance of the PrP expression in zebrafish has a more drastic effect on the early development than observed in the mammalian species studied. A further conclusion is that PrP-1 and PrP-2 serve different purposes during zebrafish embryogenesis. While early expression of PrP-1 is essential for gastrulation, expression of PrP-2 in the developing nervous system is required for the proper formation of neural structures.Citation2,Citation22,Citation32,Citation33 Interestingly, a recent study of differentiation in mouse PrP-null (KO) and WT embryonic stem cell (ESC) lines has provided strong evidence for a relationship between PRNP and several key pluripotency genes, confirming the involvement of PrP in early embryogenesis.Citation34
The amenability of zebrafish to global technologies may throw new light on prion biology. An area of intense interest has been splice variants of PRNP, “anchorless” PrP and the general question of cytoplasmic PrP. In human glioblastoma cells, two isoforms of PRNP mRNA have been reported, one of which lacks GPI-anchor domain and is expressed as a soluble intracellular protein.Citation35 In transgenic mice studies, anchorless prion protein has been shown to result in the formation of infectious material but without clinical disease, providing evidence that membrane-bound PrP was necessary for disease.Citation9 Analysis of the transcriptome derived from zebrafish embryo mRNA present at 5.3 hpf indicates splice variants of PrP-2.Citation31 This analysis shows that the 3 exon isoform probably does not possess a GPI anchor and is analogous to the isoform reported in the human glioblastoma cells (). The existence of splice variants of PrP-2 in the zebrafish embryo may add a new layer of complexity to PrP function and indicate new directions for functional studies in prion biology. Based on its expression profile and morphant phenotype, PrP-2 is probably more closely related to mammalian PRNP.Citation2,Citation29,Citation31–Citation33 The view of prion protein function may be clearer at a phylogenetic distance.
Figures and Tables
Figure 1 PrP-2 gene knockdown induced differentially expressed genes involved in neurogenesis and developmental processes in 24 hpf zebrafish embryos injected with PrP-2 morpholino. Red color indicates up and green downregulation of the genes indicated. The figure was generated by Ingenuity Pathway Analysis program (IPA; www.ingenuity.com), which clusters significantly differentially expressed genes and generates a pathway and biological function analysis. The symbols for different gene products involved are presented in the top part.
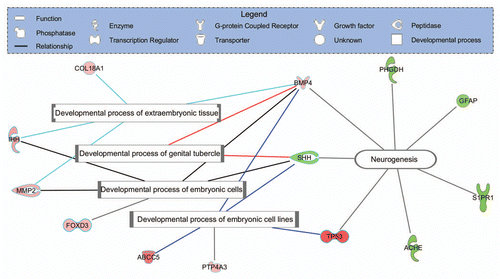
Figure 2 PrP-2 mRNA splice isoforms: At least two isoforms of prnprs3 (PrP-2) mRNA are described in zebrafish; (A) A 2 exon transcript (RefSeq: NM_001013298.1; UCSC Genome Browser on Zebrafish Jul 2010) and (B) A 3 exon transcript (ENSDART00000116357, Zv9 Ensembl release 61-Feb 2011). The latter discloses an extra intron in the C-terminus of the open reading frame leading to a frame shift, which causes the C-terminal end to switch from being hydrophobic to more hydrophilic. The figure was created using CLC Main Workbench (CLC bio, Aarhus, Denmark).
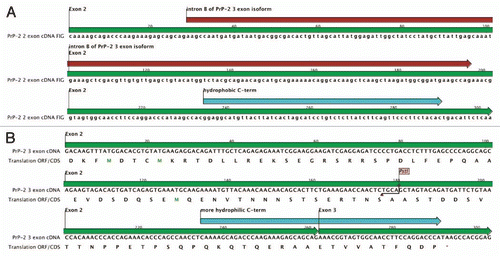
Table 1 Gene accession numbers, gene symbols, names, molecule type and fold change for differentially expressed genes involved in neurogenesis and developmental processes in 24 hpf zebrafish embryos injected with PrP-2 morpholino
Acknowledgments
The authors thank Michael Tranulis, Norwegian School of Veterinary Science, for critical reading of the manuscript.
References
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136 - 144
- Nourizadeh-Lillabadi R, Seilø Torgersen J, Vestrheim O, König M, Aleström P, Syed M. Early embryonic gene expression profiling of zebrafish prion protein (Prp2) morphants. PLoS One 2010; 5:13573
- Prusiner SB. Prusiner SB. An introduction to prion biology and diseases. Prion Biology and Diseases 1999; Cold Spring Harbor, New York Cold Spring Harbor Laboratory Press 1 - 66
- Lasmézas CI. The transmissible spongiform encephalopathies. Rev Sci Tech 2003; 22:23 - 36
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 1992; 16:577 - 582
- Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol 1994; 8:121 - 127
- Richt JA, Kasinathan P, Hamir AN, Castilla J, Sathiyaseelan T, Vargas F, et al. Production of cattle lacking prion protein. Nat Biotechnol 2007; 25:132 - 138
- Zhu C, Li B, Yu G, Chen J, Yu H, Chen J, et al. Production of Prnp-/- goats by gene targeting in adult fibroblasts. Transgenic Res 2009; 18:163 - 171
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 2005; 308:1435 - 1439
- Tveit H, Lund C, Olsen CM, Ersdal C, Prydz K, Harbitz I, et al. Proteolytic processing of the ovine prion protein in cell culture. Biochem Biophys Res Commun 2005; 337:232 - 240
- Vilette D, Andréoletti O, Archer F, Madelaine MF, Vilotte JL, Lehmann S, et al. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc Natl Acad Sci USA 2001; 98:4055 - 4059
- Greenwood AD, Horsch M, Stengel A, Vorberg I, Lutzny G, Maas E, et al. Cell line dependent RNA expression profiles of prion-infected mouse neuronal cells. J Mol Biol 2005; 349:487 - 500
- Riemer C, Neidhold S, Burwinkel M, Schwarz A, Schultz J, Krätzschmar J, et al. Gene expression profiling of scrapie-infected brain tissue. Biochem Biophys Res Commun 2004; 323:556 - 564
- Xiang W, Windl O, Wünsch G, Dugas M, Kohlmann A, Dierkes N, et al. Identification of differentially expressed genes in scrapie-infected mouse brains by using global gene expression technology. J Virol 2004; 78:11051 - 11060
- Brown AR, Rebus S, McKimmie CS, Robertson K, Williams A, Fazakerley JK. Gene expression profiling of the preclinical scrapie-infected hippocampus. Biochem Biophys Res Commun 2005; 223:86 - 95
- Satoh J, Yamamura T. Gene expression profile following stable expression of the cellular prion protein. Cell Mol Neurobiol 2004; 24:793 - 814
- Austbø L, Kampmann A, Müller-Ladner U, Neumann E, Olsaker I, Skretting G. Identification of differentially expressed genes in ileal Peyer's patch of scrapie-infected sheep using RNA arbitrarily primed PCR. BMC Vet Res 2008; 28:4 - 12
- Bossers A, Harders F, Smits M, van Keulen L, van Zijderveld F. Identification of early natural scrapie-specific gene expression changes in Peyer's patches of sheep using a generated sheep cDNA microarray. Prion Between fundamentals and society's needs, Düsseldorf 2005; 10 19–21 220
- Almeida LM, Basu U, Khaniya B, Taniguchi M, Williams JL, et al. Gene expression in the medulla following oral infection of cattle with bovine spongiform encephalopathy. J Toxicol Environ Health A 2011; 74:110 - 126
- Chadi S, Young R, Le Guillou S, Tilly G, Bitton F, Martin-Magniette ML, et al. Brain transcriptional stability upon prion protein-encoding gene invalidation in zygotic or adult mouse. BMC Genomics 2010; 11:448
- Aguzzi A. Prion toxicity: All sail and no anchor. Science 2005; 308:1420 - 1421
- Malaga-Trillo E, Solis GP, Schrock Y, Geiss C, Luncz L, et al. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol 2009; 7:55
- Teh C, Parinov S, Korzh V. New ways to admire zebrafish: progress in functional genomics research methodology. Bio Techniques 2005; 38:897 - 906
- Aleström P, Holter JL, Nourizadeh-Lillabadi R. Zebrafish in functional genomics and aquatic biomedicine. Trends Biotechnol 2006; 24:15 - 21
- Skromne I, Prince VE. Current perspectives in zebrafish reverse genetics: Moving forward. Dev Dyn 2008; 237:861 - 882
- Foley JE, Maeder ML, Pearlberg J, Joung JK, Peterson RT, Yeh JR. Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat Protoc 2009; 4:1855 - 1867
- Newman M, Musgrave FI, Lardelli M. Alzheimer disease: Amyloidogenesis, the presenilins and animal models. Biochimica et Biophysica Acta 2007; 1772:285 - 297
- Bretaud S, Allen C, Ingham PW, Bandmann O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease. J Neurochem 2007; 100:1626 - 1635
- Cotto E, André M, Forgue J, Fleury HJ, Babin PJ. Molecular characterization, phylogenetic relationships and developmental expression patterns of prion genes in zebrafish (Danio rerio). FEBS J 2005; 272:500 - 513
- Miesbauer M, Bamme T, Riemer C, Oidtmann B, Winklhofer KF, et al. Prion protein-related proteins from zebrafish are complex glycosylated and contain a glycosylphosphatidylinositol anchor. Biochem Biophys Res Commun 2006; 341:218 - 224
- Aanes H, Winata CL, Lin CH, Chen JP, Srinivasan KG, Lee SGP, et al. Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition 2011. Genome Res In press
- Málaga-Trillo E, Sempou E. PrPs: Proteins with a purpose: Lessons from the zebrafish. Prion 2009; 3:129 - 133
- Chiesa R, Harris DA. Fishing for prion protein function. PLoS Biol 2009; 7:1000075; http://dx.doi.org/10.1371/journal.pbio.1000075
- Miranda A, Pericuesta E, Ramı'rez MA', Gutierrez-Adan A. Prion protein expression regulates embryonic stem cell pluripotency and differentiation. PLoS One 2001; 6:18422; http://dx.doi.org/10.1371/journal.pone.0018422
- Kikuchi Y, Kakeya T, Nakajima O, Sakai A, Ikeda K, Yamaguchi N, et al. Hypoxia induces expression of a GPI-anchorless splice variant of the prion protein. FEBS J 2008; 275:2965 - 2976