Abstract
We recently developed a new in vitro amplification technology, designated “real-time quaking-induced conversion (RT-QUIC)”, for detection of the abnormal form of prion protein (PrPSc) in easily accessible specimens such as cerebrospinal fluid (CSF). After assessment of more than 200 CSF specimens from Japanese and Australian patients, we found no instance of a false positive, and more than 80% accuracy for the correct diagnosis of sporadic Creutzfeldt–Jakob disease (sCJD). Furthermore, the RT-QUIC can be applied to other prion diseases, including scrapie, chronic wasting disease (CWD), and bovine spongiform encephalopathy (BSE), and is able to quantify prion seeding activity when combined with an end-point dilution of samples. These results indicate that the RT-QUIC, with its high sensitivity and specificity, will be of great use as an early, rapid and specific assay for prion diseases.
Diagnosis of Creutzfeldt-Jakob Disease: The Current Situation
Human prion diseases, including Creutzfeldt-Jakob disease (CJD), are incurable neurodegenerative disorders characterized by progressive spongiform changes and the accumulation of abnormal prion protein (PrPSc) in the central nervous system.Citation1 The majority of CJD cases (approximately 85%) are sporadic in nature, but the remaining cases comprise genetic and infectious forms. Iatrogenic CJD is the consequence of inadvertent transmission during medical procedures in which sporadic CJD (sCJD)-contaminated tissues or explants (such as dura mater and pituitary hormones) or surgical instruments were used.Citation2 Variant CJD (vCJD) is primarily a zoonosis that arose from contamination of the human food chain by bovine spongiform encephalopathy (BSE), although secondary transmission of vCJD by blood transfusion has also been reported in reference Citation3. Hence, by adopting additional infection control measures as appropriate, an early and accurate diagnosis of CJD would help to lessen the possibility of iatrogenic transmission and lead the way to timely therapeutic interventions. However, the definitive ante-mortem confirmation of CJD currently requires the presence of typical neuropathology together with the demonstration of PrPSc in specimens obtained by biopsy, the practice of which is often precluded both by the invasiveness of the procedure and the risks it poses to medical care staff. Thus, the highly sensitive detection of PrPSc in accessible body fluids such as cerebrospinal fluid (CSF) and blood can be expected to constitute a most valuable means for the early and specific diagnosis of CJD. However, because the concentration of PrPSc in these specimens is likely to be very low, one of the most promising approaches would be to develop an efficient amplification of PrPSc in vitro.Citation4,Citation5 Indeed, several assays, including protein misfolding cyclic amplification (PMCA),Citation6,Citation7 the amyloid seeding assay (ASA),Citation8 and quaking-induced conversion (QUIC),Citation9,Citation10 have previously been reported to permit the sensitive detection of PrPSc in animal and human brain specimens. Nonetheless, early attempts at ultrasensitive PrPSc detection in accessible body fluids were unsuccessful in human prion diseases. For this reason, we initiated studies aimed at establishing a highly sensitive assay for the detection of prion in human CSF.
Establishment of Real-Time QUIC (RT-QUIC)
In the QUIC assays, soluble recombinant PrP (rPrP-sen) expressed in E. coli is used as a substrate to amplify the minute amounts of PrPSc. Using a dedicated shaker, the reaction is enhanced by vigorous intermittent shaking which induces rPrP-sen to aggregate and form fibrils.Citation9 One of the advantages in QUIC is that shaking/agitation can be performed more easily and consistently than sonication, which has the problem of varied delivery of vibrational energy to the samples. On the other hand, further improvement in the rapidity and practicality of this method was required in order for it to become useful in the diagnosis of prion diseases, because the initial standard format of QUIC (S-QUIC) required a time-consuming western blot. Thus, we combined QUIC technology with thioflavin T (ThT) fluorescence dye, to monitor amyloid formation, in order to minimize the time necessary for the detection of protease-resistant rPrP fibrils (rPrP-res). We first determined if the shaking of our fluorescence microplate reader could induce PrPSc-dependent rPrP-res (rPrP-resSc) formation in a buffer containing 0.05–0.1% SDS, as in the S-QUIC. Human rPrP-sen (rHuPrP-sen) and CJD brain homogenate (BH) were used as a substrate and a seed, respectively, for the QUIC reaction. Unexpectedly, we did not observe rPrP-resSc formation or an elevation in the ThT fluorescence using our microplate reader (unpublished data). Our explanation for this is that the shaking power of the microplate reader was not strong enough to elicit the QUIC reactions in the presence of SDS. SDS tends to cause fibrils to stack and stabilize rPrP-res polymers as a result. In fact, we observed that fibrils formed in the presence of SDS were much larger and thicker than those formed in its absence. Taken together, sonication in PMCA or vigorous shaking in S-QUIC seems to be required as a means of fragmenting the rPrP-res polymers formed in the presence of SDS.
We then tested whether rPrP-resSc formation was induced when guanidine-HCl (GdnHCl) was added, because it has been thought that GdnHCl was required for the conversion of PrP-sen to PrP-res in a cell-free system.Citation11 Somewhat unexpectedly, we observed rPrP-resSc formation even in the absence of GdnHCl. In contrast, the negative control reactions without seed and in the absence of GdnHCl exhibited a marked delay in spontaneous rPrP-res (rPrP-resspon) formation.Citation12 For this reason, use of a GdnHCl-free buffer can dramatically reduce the risk of false-positive reactions and enhance the sensitivity of the method.
Shaking/agitation is considered to cause several facilitatory effects on the QUIC reaction. One is that a partial unfolding of a portion of the rPrP-sen is induced by increasing the air-water interface through which a denaturing boundary between the hydrophobic air and hydrophilic water is formed.Citation13 Next, shaking/agitation enhances the interaction between rPrP-sen and PrPSc, and the fragmentation of rPrP-res polymers.Citation14 The energetic barrier of spontaneous fibril formation is likely to be higher than that of seed-dependent fibril formation or elongation, because spontaneous formation initially necessitates nucleation as the rate-limiting step.Citation15
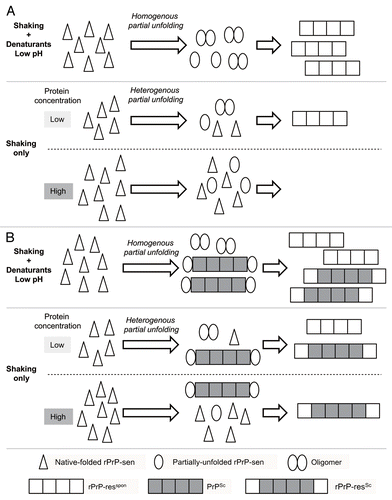
Meanwhile, the extent of the partial unfolding of rPrP-sen by shaking alone is assumed to be more heterogeneous than that in the presence of GdnHCl, probably because the air-water interfaces are unequally distributed in the reaction mixture (). In contrast, the addition of GdnHCl accelerates the nucleation rate, resulting in an increase in the rate of spontaneous fibril formation. Of note, we observed that there was an inverse correlation between the rate of rPrP-res formation and the concentration of rPrP-sen substrate.Citation12 It has been reported that the aggregation rate of several other proteins is inversely correlated with the concentration of substrate protein in a denaturant-free buffer with shaking.Citation16,Citation17 Conversely, previous studies using cell-free conversionCitation18 and rPrP fibril formation,Citation19–Citation21 respectively, in the presence of denaturant or at low pH, have shown that the rate of PrP-res formation was directly proportional to the PrP-sen concentration. This seeming contradiction can be explained again by the difference in the denaturation status of PrP-sen under various conditions. We hypothesized that heterogenous denaturation of the substrate protein in a denaturant-free buffer with shaking is a major cause of the inverse correlation ().
We examined the effect of pH, and the concentrations of rHuPrP-sen and salt, on QUIC reactions in a GdnHCl-free buffer. We found that the presence of NaCl is essential for rPrP-res formation and the sensitivity of this method was maximal at 500 mM NaCl at pH 7.Citation12 The requirement for NaCl in the formation of rPrP-res is compatible with previous studies, which have shown that salt is required for cell-free conversion in the absence of GdnHClCitation22 and the maintenance of a protease-resistant PrPSc conformation.Citation23
We named this new assay “real-time QUIC (RT-QUIC)” by analogy with real-time PCR. The RT-QUIC enabled us to measure up to 96 replicates at a time, obtain the results immediately, and is potentially safer than S-QUIC or PMCA because the prions are sealed within a 96-well plate throughout the entire procedure.
Application of RT-QUIC to Diagnostic Tests for Human Prion Diseases
CJD has been categorized into six molecular subtypes (MM1, MM2, MV1, MV2, VV1, VV2) on the basis of whether methionine (M) or valine (V) is present at codon 129 of the gene encoding prion protein, combined with the profile of PrPSc (type 1 or type 2).Citation24 We evaluated the detection limit of MM1- and MM2-sCJD brain homogenate using RT-QUIC. The minimum amount of PrPSc in the brains detectable by RT-QUIC was around ∼1 fg (10−15 g). To determine the applicability of RT-QUIC in the clinical diagnosis of sCJD, we compared the RT-QUIC seeding activity in CSF samples from patients with sCJD and patients without sCJD but with other neurodegenerative diseases such as Alzheimer disease. We decided upon CSF as the specimen because CSF is routinely used in the assessment of many neurological disorders. Moreover, CSF is likely to contain more PrPSc and fewer impurities than blood. Examining more than 200 CSF specimens from Japanese and Australian patients, we demonstrated that RT-QUIC has greater than 80% sensitivity and absolute specificity for the detection of PrPSc in the CJD-positive CSF samples.Citation12 Until now, diagnostic investigations to evaluate suspected sCJD, although of proven utility, have relied upon non-specific bio-markers, such as the detection of 14-3-3 proteins in the CSF.Citation25–Citation27 The sensitivity of RT-QUIC was equivalent to and the specificity was much higher than that achieved by 14-3-3 protein measurement. Thus, the RT-QUIC provides a valuable novel means for the antemortem diagnosis of sCJD. Although most of the CSF samples we tested were 129MM, 3/4 129VV and 2/2 129MV CSF samples were positive, suggesting that RT-QUIC using 129M rHuPrP-sen as a substrate is equally valuable in all genetic subtypes of sCJD. Additionally, we recently found that RT-QUIC is potentially useful in the diagnosis of genetic human prion diseases, including Gerstmann-Straussler-Schenker disease (GSS) and fatal familial insomnia (FFI) (manuscript in preparation). While the conversion between PrP-sen and PrPSc with identical sequences is generally thought to be efficient, our findings suggest that the degree of sequence correspondence between substrate and seed can vary in the RT-QUIC reactions. In support of this concept, we observed that hamster or bovine rPrP-sen can be actively converted into rPrP-res when seeded with sCJD-PrPSc, albeit with about one log reduction in the detection limit (unpublished data). Furthermore, Orru et al. reported that the use of hamster-sheep chimera rPrP-sen provided for greater sensitivity and less spontaneous fibril formation than was observed with the homologous rHuPrP-sen in the RT-QUIC seeded with vCJD brain homogenate.Citation28 These results raise the possibility that rPrP-sen may also react to fibrils consisting of other proteins such as beta-amyloid, possibly resulting in a decrease in the specificity of the assay. However, we have yet to experience a single false positive in the RT-QUIC among hundreds of CSF specimens from non-CJD neurodegenerative diseases, including Alzheimer disease, we have tested. Moreover, no increase in ThT fluorescence was observed in the presence of beta-amyloid fibrils artificially formed in vitro (unpublished data). Nevertheless, further studies will be required to completely eliminate the possibility of false positives in the clinical setting. Additionally, the elucidation of the mechanism of rPrP-resSc formation in the RT-QUIC, including the degree of sequence correspondence, would lead to a better understanding of the molecular basis of prion propagation.
Further Progress in RT-QUIC Technology
Recently, Caughey's group demonstrated that our RT-QUIC could be successfully applied to the detection of hamster and sheep scrapie, deer chronic wasting disease (CWD) and vCJD.Citation28,Citation29 Additionally, our team has been able to detect BSE at a sensitivity equivalent to that of sCJD (manuscript in preparation). In addition, the RT-QUIC can rapidly determine the relative prion concentration when used in combination with end-point dilution analysis.Citation29 In another very recent study, Caughey's team showed that enrichment of PrPSc in plasma by immunoprecipitation employing the PrP aggregate-specific monoclonal IgM antibody 15B3 greatly enhances the sensitivity of RT-QUIC, especially when coupled with a substrate replacement step.Citation28 Together, these studies demonstrated the wide-ranging application of RT-QUIC to clinical and basic research on human and animal prion diseases.
Figures and Tables
Figure 1 Hypothetical models for the inverse correlation between rPrP-sen concentration and fibril formation in a denaturant-free buffer with shaking in the absence (A) or presence of host-derived PrPSc (B). Homogeneous partial-unfolding of rPrP-sen is induced in the presence of denaturant or at low pH, leading to an increase of oligomer formation. In contrast, a heterogeneous denaturation status of rPrP-sen is presumed to be inversely proportional to the concentration in a denaturant-free buffer with shaking, resulting in a reduction of oligomer formation. It remains to be determined whether native-folded rPrP-sen can bind to PrPSc or rPrP-res polymers.
References
- Prusiner SB. Prions. Proc Natl Acad Sci USA 1998; 95:13363 - 13383
- Hamaguchi T, Noguchi-Shinohara M, Nozaki I, Nakamura Y, Sato T, Kitamoto T, et al. The risk of iatrogenic Creutzfeldt-Jakob disease through medical and surgical procedures. Neuropathology 2009; 29:625 - 631
- Ironside JW. Variant Creutzfeldt-Jakob disease. Haemophilia 5:175 - 180
- Castilla J, Saa P, Morales R, Abid K, Maundrell K, Soto C. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol 2006; 412:3 - 21
- Atarashi R. Recent advances in cell-free PrPSc amplification technique. Protein Pept Lett 2009; 16:256 - 259
- Saa P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science 2006; 313:92 - 94
- Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods 2007; 4:645 - 650
- Colby DW, Zhang Q, Wang S, Groth D, Legname G, Riesner D, et al. Prion detection by an amyloid seeding assay. Proc Natl Acad Sci USA 2007; 104:20914 - 20919
- Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods 2008; 5:211 - 212
- Orru CD, Wilham JM, Hughson AG, Raymond LD, McNally KL, Bossers A, et al. Human variant Creutzfeldt-Jakob disease and sheep scrapie PrP(res) detection using seeded conversion of recombinant prion protein. Protein Eng Des Sel 2009; 22:515 - 521
- Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, Lansbury PT, et al. Cell-free formation of protease-resistant prion protein. Nature 1994; 370:471 - 474
- Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 2011; 17:175 - 178
- Toth SI, Smith LA, Ahmed SA. Extreme sensitivity of botulinum neurotoxin domains towards mild agitation. J Pharm Sci 2009; 98:3302 - 3311
- Collins SR, Douglass A, Vale RD, Weissman JS. Mechanism of prion propagation: amyloid growth occurs by monomer addition. PLoS Biol 2004; 2:321
- Lansbury P Jr, Caughey B. The chemistry of scrapie infection: implications of the ‘ice 9’ metaphor. Chem Biol 1995; 2:1 - 5
- Sluzky V, Tamada JA, Klibanov AM, Langer R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc Natl Acad Sci USA 1991; 88:9377 - 9381
- Treuheit MJ, Kosky AA, Brems DN. Inverse relationship of protein concentration and aggregation. Pharm Res 2002; 19:511 - 516
- Caughey B, Kocisko DA, Raymond GJ, Lansbury P Jr. Aggregates of scrapie-associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem Biol 1995; 2:807 - 817
- Baskakov IV, Bocharova OV. In vitro conversion of mammalian prion protein into amyloid fibrils displays unusual features. Biochemistry 2005; 44:2339 - 2348
- Jain S, Udgaonkar JB. Evidence for stepwise formation of amyloid fibrils by the mouse prion protein. J Mol Biol 2008; 382:1228 - 1241
- Stohr J, Weinmann N, Wille H, Kaimann T, Nagel-Steger L, Birkmann E, et al. Mechanisms of prion protein assembly into amyloid. Proc Natl Acad Sci USA 2008; 105:2409 - 2414
- Horiuchi M, Caughey B. Specific binding of normal prion protein to the scrapie form via a localized domain initiates its conversion to the protease-resistant state. EMBO J 1999; 18:3193 - 3203
- Nishina K, Jenks S, Supattapone S. Ionic strength and transition metals control PrPSc protease resistance and conversion-inducing activity. J Biol Chem 2004; 279:40788 - 40794
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 1999; 46:224 - 233
- Hsich G, Kenney K, Gibbs CJ, Lee KH, Harrington MG. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Engl J Med 1996; 335:924 - 930
- Zerr I, Bodemer M, Gefeller O, Otto M, Poser S, Wiltfang J, et al. Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol 1998; 43:32 - 40
- Satoh K, Tobiume M, Matsui Y, Mutsukura K, Nishida N, Shiga Y, et al. Establishment of a standard 14-3-3 protein assay of cerebrospinal fluid as a diagnostic tool for Creutzfeldt-Jakob disease. Lab Invest 2010; 90:1637 - 1644
- Orru CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, et al. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio 2011; 2:00078-11
- Wilham JM, Orru CD, Bessen RA, Atarashi R, Sano K, Race B, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog 2011; 6:1001217