Abstract
The soluble cellular prion protein (PrPC) is best known for its association with prion disease (PrD) through its conversion to a pathogenic insoluble isoform (PrPSc). However, its deleterious effects independent of PrPSc have recently been observed not only in PrD but also in Alzheimer disease (AD), two diseases which mainly affect cognition. At the same time, PrPC itself seems to have broad physiologic functions including involvement in cognitive processes. The PrPC that is believed to be soluble and monomeric has so far been the only PrP conformer observed in the uninfected brain. In 2006, we identified an insoluble PrPC conformer (termed iPrPC) in uninfected human and animal brains. Remarkably, the PrPSc-like iPrPC shares the immunoreactivity behavior and fragmentation with a newly-identified PrPSc species in a novel human PrD termed variably protease-sensitive prionopathy. Moreover, iPrPC has been observed as the major PrP species that interacts with amyloid β (Aβ) in AD. This article highlights evidence of PrP involvement in two putatively beneficial and deleterious PrP-implicated pathways in cognition, and hypothesizes first, that beneficial and deleterious effects of PrPC are attributable to the chameleon-like conformation of the protein and second, that the iPrPC conformer is associated with PrD and AD.
Introduction
Prion diseases (PrDs) are a group of fatal neurodegenerative disorders in which cognitive decline and dementia constitute the early predominant clinical manifestations.Citation1 In contrast to Alzheimer disease (AD), which is the most common cause of dementia in adults, and characterized by the deposition of noninfectious amyloid-β (Aβ) plaques consisting of a peptide containing 42 amino acids (termed Aβ42) generated from the amyloid precursor protein,Citation2 PrDs are associated with the deposition in the brain of an infectious prion protein conformer (PrPSc) that is derived from its cellular prion protein (PrPC) by a structural transition.Citation3
PrPC and PrPSc are the two major PrP conformers studied so far. They share the same primary structure but have distinct secondary structures,Citation4–Citation6 which explains the distinction in their physicochemical and biological features, physiology and pathophysiology. PrPC is monomeric, rich in α-helical structure, sensitive to proteinase K (PK)-digestion, soluble in non-denaturing detergents, non-infectious and present in both uninfected and scrapie-infected brains. PrPSc, on the other hand, is oligomeric or aggregate, rich in β-sheet structure, partially resistant to PK, insoluble in detergents, infectious and present in infected brains only. Although PrPC is the physiologic form of the protein, it has been well documented that the co-existence of PrPC and PrPSc constitutes the prerequisite for the emergence of PrDs. The distinct prion strains and phenotypes of PrDs which have been identified in animals and humans,Citation7,Citation8 are probably associated with variable conformations of PrPSc.Citation9–Citation12 Recent identification of the insoluble cellular PrP (iPrPC) in the uninfected human and animal brain raises the possibility that additional PrPC conformers in the brain may be implicated in the beneficial or deleterious effect of PrPC and that they may play a role in the pathogenesis of PrDs and other neurodegenerative disorders.Citation13
PrPC is Physiologically Involved in Human Cognitive Processes
PrPC may play a role in human cognitive processes by interacting with other proteins in synapses.Citation14 Long-term memory formation is believed to begin at synapses located on dendritic spines.Citation15 PrPC is highly concentrated at the synapse,Citation16 presynaptically and postsynaptically as well.Citation16–Citation19 PrPC significantly affected synapse formation, and the simple incubation of cultured hippocampal neurons with recombinant PrP (rPrP) in vitro induced rapid elaboration of axons and dendrites, along with increases in the number of synaptic contacts.Citation20 In fact, a dominant synaptic impairment is often observed in PrDs,Citation21 impairment which may result from the conversion of PrPC into PrPSc.
PrP deletion impaired long-term potentiation (LTP) and decreased a fast inhibition involving GABA-A receptors.Citation22,Citation23 Also, it has been shown that PrP-knockout mice developed either an age-dependent impairment in memory consolidationCitation24 or in hippocampus-dependent spatial learning.Citation25 These deficits were reversed by re-expression of PrPC.Citation26 On the other hand, mice overexpressing PrP exhibited supra-physiologic responses, and a positive correlation was evident between the expression level of PrPC and the overall strength of glutamatergic transmission in the hippocampus.Citation26 These observations strongly suggest that PrPC constitutes the components of cognitive processes in synapses. While the detailed molecular events underlying the effect of PrPC on cognitive processes remain unclear, it has been observed that blocking the association of PrPC with stress-inducible protein 1 and with laminin by antibodies adversely influences memory.Citation27,Citation28 Therefore, under normal conditions, the intact interaction of PrPC with other proteins in the synapses may be necessary to maintain normal cognitive functions through a beneficial PrPC-implicating pathway. Finally, recent findings that overexpression of PrPC inhibits β-secretase cleavage of APP and Aβ formation, and that Aβ formation increases in manipulated PrP-deficient cells and in PrP-knockout mouse brains,Citation29 also suggest that PrPC plays a protective role against cognitive impairment by AD.
PrPC is Pathologically Involved in Human Cognitive Processes
At the same time, however, several lines of evidence indicate that PrPC may also be implicated in a pathway which adversely affects cognitive performance. Depletion of PrPC in mice with early prion infection reversed spongiform change and prevented the emergence of clinical symptoms and neuronal loss.Citation30 Moreover, the early hippocampal pathology, including impaired synaptic responses, preceded the accumulation of PrPSc deposits and the pathologic changes rapidly reversed after PrPC depletion.Citation31 Similarly, blocking certain PrPC areas by anti-PrP antibodies, or by reducing and alkylating reagents, to diminish the availability of PrPC, prevented PrP conversion and even cured prion infection in vitro and in vivo.Citation32–Citation34 Cumulatively, these observations are entirely consistent with the remarkable finding that PrPC-knockout mice are resistant to prion infection.Citation35 Therefore, PrPSc as well as PrPC are necessary for the pathogenesis of PrDs.
The detrimental face of PrPC also has been reported in other abnormal conditions. For example, its potential role in the pathogenesis of AD has recently come to light. PrPC polymorphism, either methionine (M) or valine (V) at residue 129 (129M or 129V), may modulate the number of Aβ deposits during aging.Citation36 Remarkably, of the 225,000 proteins that were screened in a cell model, only PrP-expressing cells were found to strongly support the binding of soluble Aβ42 oligomers, the neurotoxic species; moreover, in mouse brain slices, Aβ42 oligomers suppressed LTP in CA1 hippocampal neurons in a PrPC-dependent manner.Citation37 This suppression was completely abolished by deletion of murine PrP residues 32–106 or significantly reduced by an antibody against PrP93-109. AD transgenic mice with intact PrPC expression exhibited deficits in spatial learning and memory, whereas no impairment of spatial learning and memory was detected in mice lacking PrPC.Citation38 These findings were not reproduced in different animal models by other laboratories.Citation39–Citation41 However, Strittmatter and co-workers' findings were consistent with studies in which the anti-PrP antibodies administrated peritoneally or intracerebroventricularly either improved AD mouse deficits in cognitive learning or prevented the inhibition of LTP by Aβ oligomers.Citation42,Citation43 Strikingly, PrPC was observed to mediate neurotoxic signaling of β-sheet-rich conformers including not only PrPSc and Aβ oligomers but also yeast prions and designed β-peptides in cell models.Citation44 The PrPC-mediated neurotoxic signaling arises from the binding of these β-sheet-rich conformers to the intrinsically disordered N-terminal PrP domain between residues 27 and 89. PrP 27–89 includes the octapeptide repeat region that contains the Aβ42-specific binding sites.Citation45 In addition, several new studies also suggest that PrP may be involved in AD beneficially or deleteriously.Citation45–Citation49
Accordingly, PrPC may be implicated in the impairment of memory and cognition via an unknown pathway which is triggered by abnormal protein aggregation such as PrPSc in PrDs and Aβ oligomers in Alzheimer disease ().Citation50 As a result, under pathologic conditions, blocking or depleting PrPC becomes an important, if not exclusive strategy for restoring cognitive functioning. In PrDs, some or all of PrPC converts to PrPSc, which may not only damage the favorable pathway by a decrease in PrPC but also trigger the unfavorable pathway by the newly formed PrPSc. However, the molecular mechanisms underlying prion-related synaptic changes remain unclear. Nevertheless, the above evidence suggests that an unfavorable PrPC-implicated pathway exists in human cognitive processes, especially in PrDs and Alzheimer disease. Therefore, it would be important to determine which proteins are involved in the downstream of the Aβ-PrPC or PrPSc-PrPC pathway ().
The Beneficial and Deleterious Effects of PrPC and its Chameleon-Like Conformation
What is the molecular basis of the beneficial and deleterious effects of PrPC? One striking structural feature of PrP is its chameleon-like conformation,Citation11,Citation12 which may be structurally associated with beneficial and deleterious effects of the protein.Citation50 In aqueous solutions, rPrP formed a variety of conformations including pH-dependent α-helical conformations and thermodynamically more stable conformations rich in β-sheet.Citation51 The existence of PrP folding intermediates was also indicated by hydrogen exchange experiments,Citation52 and by high pressure NMR and fluorescence spectroscopy.Citation53,Citation54 In addition to a monomer, a β-oligomer and an amyloid fibril,Citation55–Citation59 two additional polymeric transient intermediates were also identified during fibrillogenesis of rPrP in vitro.Citation60
The tendency of PrP to form multiple non-native isoforms rich in β-sheets in vitro, as demonstrated by biophysical studies on rPrP, may represent a unique intrinsic feature of this protein. In other words, the multiple conformational forms of rPrP may represent an intrinsic molecular spectrum of PrPC in vivo. If so, it should be expected that more PrP conformers would be present in the brain in addition to the two major conformers PrPC and PrPSc. We did indeed confirm this when we identified novel conformers which form insoluble cellular PrP aggregates and protease-resistant PrP species in uninfected human and animal brains.Citation13 Moreover, by using gel filtration we revealed that PrP in uninfected human brains is present not only in monomers but also in oligomers and large aggregates.Citation13 These new PrP conformers, which we termed iPrPC, account for ∼5–25% of total PrP including full-length and N-terminally truncated forms; and a portion of iPrPC is resistant to PK-digestion even at 50 µg/ml.Citation13 These conformers exhibit high affinity for the gene 5 protein (g5p), a single-stranded DNA-binding protein which can also specifically bind to PrPSc but not to PrPC.Citation13,Citation61 Notably, cytosolic PrP aggregates were also observed in pancreatic beta-cells of uninfected rats in response to hyperglycemia.Citation62 By differential SDS solubility assay, PrPC species with either lower or higher solubility were also differentiated in the brain homogenates of non-infected humans, sheep and cattle.Citation63 Although the iPrPC molecule possesses PrPSc-like physicochemical properties—for instance, insolubility in non-denaturing detergents, a strong tendency to form aggregates and resistance to PK-treatment—it is unclear whether these small amounts PrPC aggregates acquire infectivity in the normal human brain. Indeed, like other PrPSc-directed antibodies,Citation64–Citation66 g5p captures not only infectious PrPSc but also non-infectious PrP aggregates or acid-denatured PrP species (unpublished data). In our laboratory we are investigating the infectivity of iPrPC using both animal and cell models. Finally, the heterogeneity of PrP has been observed in its glycosylation and endogenous fragmentations,Citation67,Citation68 which may also contribute to the various functions of the protein.
Insoluble PrPC and Prion Disease
Since iPrPC possesses PrPSc-like physicochemical properties, there is a possibility that iPrPC is an intermediate between PrPC and PrPSc () or silent PrPSc.Citation13 The observation that the brains of bigenic mice are capable of clearing prions led to the proposition that the normal brain contains low levels of PrPSc, which play a role in cellular metabolism.Citation69 Therefore, it is possible that under normal circumstances, despite the presence of a small amount of PrPSc, the brain can nevertheless maintain equilibrium between the formation and clearance of this PrPSc. Since this small amount of PrPSc does not induce a neurodegenerative disorder, presumably it is in a silent state. However, significant increases in levels of the silent prions induced by infection, PrP mutation, or unknown causes may trigger PrDs (). Fascinatingly, using protein misfolding cyclic amplification (PMCA), Barria and co-workers generated a new infectious prion without adding exogenous PrPSc seeds,Citation70 which indicates the possibility that PMCA replicated a PrPSc intermediate existing in the brain homogenate, or that the silent prion was activated by the sonication-incubation cycles involved in the PMCA process.
An alternative hypothesis is that iPrPC is a conformer which, when it increases induces an atypical form of PrDs. In fact, Westaway et al. discovered a novel neurologic syndrome in Tg mice overexpressing wild-type PrP, in which degeneration of skeletal muscle, peripheral nerves and the central nervous system was dependent on transgene dosage.Citation71 The increased amounts of wild-type PrPC might form aggregates that induce degeneration. Indeed, Chiesa et al. revealed that homozygous Tg mice overexpressing wild-type PrP at ∼10-fold but not hemizygous mice overexpressing wild-type PrP at ∼5-fold developed a spontaneous neurodegenerative disorder manifesting tremor and paresis.Citation72 Nevertheless, punctate PrP deposits and abnormally enlarged synaptic terminals with a dramatic proliferation of membranous structures were observed in both types of mice. Interestingly, the overexpressed PrP assembled into insoluble aggregates with mild PK-resistance but acquired no infectivity.Citation72 By using transgenic flies, Fernandez-Funez et al. demonstrated that misfolding and neurotoxicity of wild-type PrP are sequence-dependent: Hamster PrP formed larger amounts of PrP aggregates and induced spongiform degeneration, whereas rabbit PrP formed only very smaller amounts of PrP aggregates and did not induce spongiform degeneration.Citation73 Remarkably, the same study also found that although small amounts of PrP aggregates were also detected in young flies (day 1) expressing hamster PrP, spongiform degeneration was not evident. Therefore, the small amounts of PrP aggregates were unable to induce spongiform degeneration. Spongiform degeneration only occurred in older flies (day 30) when the levels of PrP aggregates increased.
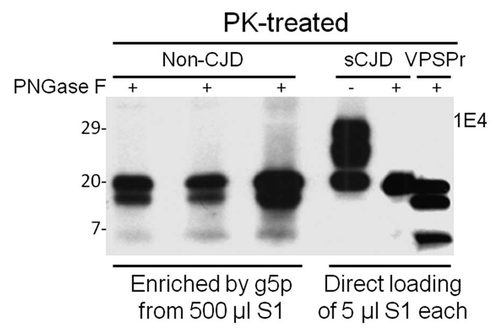
The small amount of PK-resistant PrP we identified in uninfected human brains exhibited a peculiar immunoreactivity behavior: higher affinity for 1E4 but poor affinity for 3F4.Citation13 The two antibodies have adjacent epitopes on PrP.Citation74,Citation75 The same immunoreactivity behavior has also been observed in a new PrPSc species which we recently identified in a novel human PrD termed variably protease-sensitive prionopathy (VPSPr).Citation76,Citation77 VPSPr involves an abnormal PrP with peculiar glycosylation and fragmentation and exhibits clinical features similar to those of non-Alzheimer dementias: in particular, frontotemporal dementia, diffuse Lewis body disease and normal pressure hydrocephalus.Citation76–Citation78 The molecular hallmark of VPSPr is 1E4-detected pathogenetic PK-resistant PrPSc with a ladder-like electrophoretic profile.Citation76,Citation77 PrPSc from VPSPr exhibits immunoreactivity behavior and three PK-resistant core fragments similar to those of iPrPC (). These similarities suggest that they also share a common molecular metabolic pathway (). Like sCJD, VPSPr affects all patients regardless of three PrP genotypes defined by 129 MV-polymorphism; however, the allelic prevalence is distinct in the two diseases.Citation77 Interestingly, the amounts of PK-resistant PrPSc in VPSPr are greatly affected by the polymorphism, which has not been observed in sCJD.Citation77 Moreover, the infectivity of PrPSc from VPSPr seems to be much lower compared to that of PrPSc from sCJD. Preliminary data revealed no clinical phenotype during the normal life span of the transgenic mice expressing human PrP-129V at 6-fold inoculated with brain homogenates from cases of VPSPr-129VV.Citation79 Nevertheless, 30% of the mice exhibited peculiar PrP plaques with a distinctive topography and minimal or no spongiform degeneration. Most of these mice also had the PK-resistant PrPSc whose profile exhibited the ladder-like electrophoresis detected by 1E4. Therefore, VPSPr characterized by the deposition in the brain of iPrPC-like PrPSc represents a PrD which is distinct from classical PrDs, bearing more resemblance to other neurodegenerative diseases such as Alzheimer disease and tauopathies.Citation78 Because of the similarities between iPrPC and PrPSc from VPSPr, it would be important to investigate the possibility that VPSPr results from an increase in the amount of iPrPC ().
Insoluble PrPC and Alzheimer Disease
We recently demonstrated that iPrPC is the main species that interacts with Aβ in AD.Citation80 Because of the specific or direct binding of Aβ42 to PrP, as indicated by previous studies in reference Citation37, Citation38 and Citation81 as well as our peptide membrane array and co-immunoprecipitation of soluble PrP and Aβ,Citation80 it is most likely that PrP and Aβ42 bind directly to each other within insoluble complexes. Histologically, PrP deposits often accompany Aβ-positive plaques in AD brains.Citation82–Citation84 Although the exact biological relevance of the interaction between iPrPC and Aβ is unclear at present, it has been revealed that aggregation of one protein may facilitate aggregation of the other.Citation85 Synergistic interactions between other amyloidogenic proteins associated with neurodegeneration also have been reported to promote each other's fibrillization, amyloid deposition and formation of filamentous inclusions in transgenic mice.Citation86,Citation87 Morales et al. recently observed that an increase in the efficiency of Aβ42 aggregation in vitro was dependent on PrPSc dosage. Moreover, insoluble PrPSc aggregates also seem to facilitate Aβ42 aggregation in vivo and AD mice developed a strikingly higher load of cerebral amyloid plaques that appeared much faster in prion-infected than in uninfected mice.Citation85 Our finding that Aβ42 binds to iPrP suggests that iPrP (the PrPSc-like forms in uninfected human brains) may facilitate fibrillization of Aβ42 in AD (). If this is the case, the possibility must be considered that a significant increase in the total number of Aβ plaques observed in bigenic mice overexpressing PrPCitation86 might result from an increase in the formation of iPrP. Since the less toxic insoluble Aβ42 aggregates constitute the end products of highly toxic soluble Aβ42 oligomers, formation of the large aggregates facilitated by iPrPC may reduce the amount of Aβ42 oligomers. The decrease in the levels of toxic Aβ42 oligomers would then attenuate the cognitive impairment induced by Aβ42 oligomers in AD. As a result, iPrPC may play a protective role in AD. Given that iPrPC interacts with insoluble Aβ42, whereas soluble PrPC binds soluble Aβ42 in vivo, it is possible that distinct PrP conformers binding to different Aβ42 species thereby function either as receptors for soluble Aβ42 oligomers or as modulators of insoluble Aβ42 deposition. If this hypothesis could be proved, it would establish that the chameleon-like conformation of PrPC is coupled with its beneficial and deleterious effects. This hypothesis could be tested by intracerebrally injecting anti-PrP antibodies against either soluble or insoluble PrP species in AD animal models.
Insoluble PrPC and Long-Term Memory Storage
The iPrPC species that might be of a conformation different from PrPC may have a physiological function. A neuronal isoform of the cytoplasmic polyadenylation element binding protein (CPEB) involved in long-term memory has been demonstrated to regulate local protein synthesis by activating translationally dormant mRNA. Moreover, these events stabilized synapse-specific long-term facilitation in Aplysia.Citation88 Interestingly, Si and colleagues further demonstrated that Aplysia CPEB exhibited self-perpetuating prion-like properties in sensory neurons or in yeast.Citation89,Citation90 These studies suggest that prion-like conformational changes are indispensable for the maintenance of structural synaptic changes required for long-term memory.Citation91,Citation92 It is reasonable to assume that the conversion of soluble PrPC monomers into insoluble PrP oligomers or aggregates is associated with long-term memory storage in the normal human brain (). In contrast to PrPC, the iPrPC molecule has been demonstrated to bind to g5p, the single-stranded DNA binding protein.Citation13 Accordingly, the possibility cannot be ruled out that iPrPC binds to mRNA in vivo. The PrP gene is believed to be genetically associated with human long-term memory performance, as evidenced in the observation that 24 hours after a word list-learning task, carriers of either the polymorphism methionine/methionine (M/M) at residue 129 (129MM) or M/valine (V) (129 MV) genotype recalled 17% more information than did 129VV carriers.Citation91 Therefore, the association between PrP and human memory may be mediated by M129V polymorphism, and the 129M allele may have a beneficial effect on long-term memory. It was proposed that the impact of a putative PrP conformation, rather than the pathologic PrPSc, on long-term memory in healthy humans was related to physiologically occurring conformational changes.Citation91,Citation93 It would therefore be intriguing for us to test our hypothesis that iPrPC is the PrP species that plays a beneficial role in human cognitive processes.
Conclusions
The modern study of PrD following the discovery of PrPSc and PrPC opened an extraordinary chapter in the history of the life science, which made it understandable that PrD may be composed of both transmissible and non-transmissible forms,Citation94 possibly because of the chameleon-like conformation of PrPSc.Citation11 Similarly, it is most likely that PrPC also possesses a chameleon-like feature which is reflected not only in its conformation but also in its function. Subsequent discovery of iPrPC and the demonstration of its potential association with atypical PrD and AD may open even newer avenues in the exploration of the pathogenesis of PrD and AD, thereby facilitating identification of new targets for the therapeutics of both diseases.
Abbreviations
| AD | = | Alzheimer disease |
| CPEB | = | cytoplasmic polyadenylation element binding protein |
| g5p | = | gene 5 protein |
| iPrPC | = | insoluble cellular prion protein |
| PrD | = | prion disease |
| PrP | = | prion protein |
| PrPC | = | cellular prion protein |
| PrPSc | = | pathologic conformer of PrPc |
| rPrP | = | recombinant prion protein |
| VPSPr | = | variably protease-sensitive prionopathy |
Figures and Tables
Figure 1 Diagram of the pathway involved in prion- or Aβ-induced dysfunction of memory and cognition as well as cell death. PrPSc or Aβ oligomers impair memory and cognition, and induce cell death by binding to PrPC at the synapse but the downstream molecular events (the black box with a question mark) of the PrPSc-PrPC or PrPC-Aβ complex are unknown. The iPrPC species derived from the soluble PrPC might play a role in one or more of the following events: (1) long-term memory storage; (2) PrPSc formation in classic CJD; (3) initiating VPSPr; and (4) facilitating formation of Aβ42 fibrils in AD.
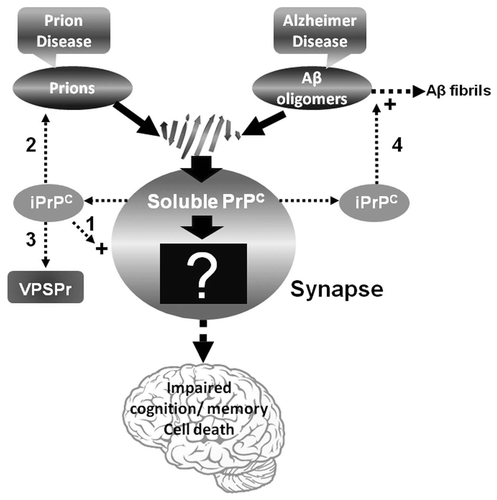
Figure 2 Comparison of PK-resistant PrP core fragments from non-CJD, VPSPr and sCJD. PrP captured with g5p from brain homogenates of three non-CJD subjects was treated with PK and PNGase F prior to SDS-PAGE and immunoblotting with anti-PrP antibody 1E4. Fifty µl of insoluble fraction (P2) equivalent to 500 µl of supernatant (S1) from a low-speed centrifuge was used for each non-CJD subject. In these highly concentrated samples from non-CJD subjects, three PK-resistant PrP (PrPres) fragments migrating at ∼20 kDa, ∼17–18 kDa and ∼6–7 kDa were detected with 1E4. But these PrPres fragments were not detectable by 3F4 (data not shown). In contrast, three PrP fragments with similar gel mobility were also detected in only 5 µl of S1 preparation from a VPSPr case (129MV) by 1E4 after treatment with PK and PNGase F. However, only one PrP band was detected in sCJD samples after PK and PNGase F treatment.
Acknowledgments
The authors are grateful to Dr. Pedro Fernandez-Funez for helpful comments. This work was supported by the National Institutes of Health R01NS062787, the University Center on Aging and Health with the support of the McGregor Foundation and the President's Discretionary Fund (Case Western Reserve University), the Alliance BioSecure and the CJD Foundation.
References
- Knight RS, Will RG. Prion diseases. J Neurol Neurosurg Psychiatry 2004; 75:36 - 42
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002; 297:353 - 356
- Prusiner SB. Prions. Proc Natl Acad Sci USA 1998; 95:13363 - 13383
- Meyer RK, McKinley MP, Bowman KA, Braunfeld MB, Barry RA, Prusiner SB. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci USA 1986; 83:2310 - 2314
- Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 1991; 30:7672 - 7680
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA 1993; 90:10962 - 10966
- Bessen RA, Marsh RF. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol 1992; 73:329 - 334
- Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG, et al. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol 1996; 39:767 - 778
- Caughey B, Raymond GJ, Bessen RA. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J Biol Chem 1998; 273:32230 - 32235
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 1998; 4:1157 - 1165
- Zou WQ, Gambetti P. Prion: the chameleon protein. Cell Mol Life Sci 2007; 64:3266 - 3270
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science 2007; 318:930 - 936
- Yuan J, Xiao X, McGeehan J, Dong Z, Cali I, Fujioka H, et al. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J Biol Chem 2006; 281:34848 - 34858
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev 2008; 88:673 - 728
- Weeber EJ, Levenson JM, Sweatt JD. Molecular genetics of human cognition. Mol Interv 2002; 2:376 - 391
- Salès N, Rodolfo K, Hässig R, Faucheux B, Di Giamberardino L, Moya KL. Cellular prion protein localization in rodent and primate brain. Eur J Neurosci 1998; 10:2464 - 2471
- Fournier JG, Escaig-Haye F, Grigoriev V. Ultra structural localization of prion proteins: physiological and pathological implications. Microsc Res Tech 2000; 50:76 - 88
- Herms J, Tings T, Gall S, Madlung A, Giese A, Siebert H, et al. Evidence of presynaptic location and function of the prion protein. J Neurosci 1999; 19:8866 - 8875
- Haeberlé AM, Ribaut-Barassin C, Bombarde G, Mariani J, Hunsmann G, Grassi J, et al. Synaptic prion protein immuno-reactivity in the rodent cerebellum. Microsc Res Tech 2000; 50:66 - 75
- Kanaani J, Prusiner SB, Diacovo J, Baekkeskov S, Legname G. Recombinant prion protein induces rapid polarization and development of synapses in embryonic rat hippocampal neurons in vitro. J Neurochem 2005; 95:1373 - 1386
- Jeffrey M, Halliday WG, Bell J, Johnston AR, MacLeod NK, Ingham C, et al. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol Appl Neurobiol 2000; 26:41 - 54
- Collinge J, Whittington MA, Sidle KC, Smith CJ, Palmer MS, Clarke AR, et al. Prion protein is necessary for normal synaptic function. Nature 1994; 370:295 - 297
- Colling SB, Collinge J, Jefferys JG. Hippocampal slices from prion protein null mice: disrupted Ca(2+)-activated K+ currents. Neurosci Lett 1996; 209:49 - 52
- Coitinho AS, Roesler R, Martins VR, Brentani RR, Izquierdo I. Cellular prion protein ablation impairs behavior as a function of age. Neuroreport 2003; 14:1375 - 1379
- Criado JR, Sánchez-Alavez M, Conti B, Giacchino JL, Wills DN, Henriksen SJ, et al. Mice devoid of prion protein have cognitive deficits that are rescued by reconstitution of PrP in neurons. Neurobiol Dis 2005; 19:255 - 265
- Carleton A, Tremblay P, Vincent JD, Lledo PM. Dose-dependent, prion protein (PrP)-mediated facilitation of excitatory synaptic transmission in the mouse hippocampus. Pflugers Arch 2001; 442:223 - 229
- Coitinho AS, Lopes MH, Hajj GN, Rossato JI, Freitas AR, Castro CC, et al. Short-term memory formation and long-term memory consolidation are enhanced by cellular prion association to stress-inducible protein 1. Neurobiol Dis 2007; 26:282 - 290
- Coitinho AS, Freitas AR, Lopes MH, Hajj GN, Roesler R, Walz R, et al. The interaction between prion protein and laminin modulates memory consolidation. Eur J Neurosci 2006; 24:3255 - 3264
- Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, et al. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc Natl Acad Sci USA 2007; 104:11062 - 11067
- Mallucci G, Dickinson A, Linehan J, Klöhn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 2003; 302:871 - 874
- Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 2007; 53:325 - 335
- Enari M, Flechsig E, Weissmann C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci USA 2001; 98:9295 - 9299
- White AR, Enever P, Tayebi M, Mushens R, Linehan J, Brandner S, Hawke S, et al. Monoclonal antibodies inhibit prion replication and delay the development of Prion disease. Nature 2003; 422:80 - 83
- Zhou X, Bi H, Wong J, Shimoji M, Yuan J, Xiao X, et al. Alkylating antitumor drug mechlorethamine conceals a structured PrP domain and inhibits in vitro prion amplification. J Toxicolo Environ Health 2011; In press
- Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, et al. Mice devoid of PrP are resistant to scrapie. Cell 1993; 73:1339 - 1347
- Berr C, Helbecque N, Sazdovitch V, Mohr M, Amant C, Amouyel P, et al. Polymorphism of the codon 129 of the prion protein (PrP) gene and neuropathology of cerebral ageing. Acta Neuropathol 2003; 106:71 - 74
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 2009; 457:1128 - 1132
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Laurén J, Gimbel ZA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci 2010; 30:6367 - 6374
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci USA 2010; 107:2295 - 2300
- Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, et al. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med 2010; 2:306 - 314
- Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature 2010; 466:3 - 4
- Chung E, Ji Y, Sun Y, Kascsak RJ, Kascsak RB, Mehta PD, et al. Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer's disease model mouse. BMC Neurosci 2010; 11:130
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, et al. Alzheimer's Disease Brain-Derived Amyloid-{beta}-Mediated Inhibition of LTP In Vivo Is Prevented by Immunotargeting Cellular Prion Protein. J Neurosci 2011; 31:7259 - 7263
- Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Müller V, Krishnan R, et al. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J 2011; 30:2057 - 2070
- Zou WQ, Xiao X, Yuan J, Puoti G, Fujioka H, Wang X, et al. Amyloid-{beta}42 Interacts Mainly with Insoluble Prion Protein in the Alzheimer Brain. J Biol Chem 2011; 286:15095 - 15105
- Westaway D, Alier K, Vergote D, MacTavish D, Mercer R, Fu W, et al. Prion proteins and the Alzheimer disease Aβ amyloid cascade. Prion 2011; 5:1
- Fleisch VC, Kaiser DM, Leighton P, Wang H, Allision WT. A novel genetic interaction between APP and PrP: notorious proteins conspire during cell adhesion in a pathway leading to CNS cell death. Prion 2011; 5:112
- Schmitz M. Influence of PrPC on the expression of Alzheimer disease-relevant proteins. Prion 2011; 5:120
- Wasilewska-Sampaio AP, Hirata P, Prado M, Brito-Moreira J, Ferreira S, Hajj G, et al. PrPC-STI1 interaction protects against synapse damage caused by soluble Aβ oligomers. Prion 2011; 5:121
- Zou WQ. Chameleon-like prion protein and human cognition. Current Topics in Biochemical Research 2010; 12:1 - 8
- Zhang H, Stockel J, Mehlhorn I, Groth D, Baldwin MA, Prusiner SB, et al. Physical studies of conformational plasticity in a recombinant prion protein. Biochemistry 1997; 36:3543 - 3553
- Nicholson EM, Mo H, Prusiner SB, Cohen FE, Marqusee S. Differences between the prion protein and its homolog Doppel: a partially structured state with implications for scrapie formation. J Mol Biol 2002; 316:807 - 815
- Kuwata K, Li H, Yamada H, Legname G, Prusiner SB, Akasaka K, et al. Locally disordered conformer of the hamster prion protein: a crucial intermediate to PrPSc?. Biochemistry 2002; 41:12277 - 12283
- Martins SM, Chapeaurouge A, Ferreira ST. Folding intermediates of the prion protein stabilized by hydrostatic pressure and low temperature. J Biol Chem 2003; 278:50449 - 50455
- Baskakov IV, Legname G, Prusiner SB, Cohen FE. Folding of prion protein to its native alpha-helical conformation is under kinetic control. J Biol Chem 2001; 276:19687 - 19690
- Morillas M, Vanik DL, Surewicz WK. On the mechanism of alpha-helix to beta-sheet transition in the recombinant prion protein. Biochemistry 2001; 40:6982 - 6987
- Lu BY, Chang JY. Isolation and characterization of a polymerized prion protein. Biochem J 2002; 364:81 - 87
- Sokolowski F, Modler AJ, Masuch R, Zirwer D, Baier M, Lutsch G, et al. Formation of critical oligomers is a key event during conformational transition of recombinant syrian hamster prion protein. J Biol Chem 2003; 278:40481 - 40492
- Baskakov IV, Legname G, Gryczynski Z, Prusiner SB. The peculiar nature of unfolding of the human prion protein. Protein Sci 2004; 13:586 - 595
- Baskakov IV, Legname G, Baldwin MA, Prusiner SB, Cohen FE. Pathway complexity of prion protein assembly into amyloid. J Biol Chem 2002; 277:21140 - 21148
- Zou WQ, Zheng J, Gray DM, Gambetti P, Chen SG. Antibody to DNA detects scrapie but not normal prion protein. Proc Natl Acad Sci USA 2004; 101:1380 - 1385
- Strom A, Wang GS, Reimer R, Finegood DT, Scott FW. Pronounced cytosolic aggregation of cellular prion protein in pancreatic beta-cells in response to hyperglycemia. Lab Invest 2007; 87:139 - 149
- Kuczius T, Karch H, Groschup MH. Differential solubility of prions is associated in manifold phenotypes. Mol Cell Neurosci 2009; 42:226 - 233
- Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, Zou WQ, et al. A prion protein epitope selective for the pathologically misfolded conformation. Nat Med 2003; 9:893 - 899
- Biasini E, Seegulam ME, Patti BN, Solforosi L, Medrano AZ, Christensen HM, et al. Non-infectious aggregates of the prion protein react with several PrPSc-directed antibodies. J Neurochem 2008; 105:2190 - 2204
- Biasini E, Tapella L, Mantovani S, Stravalaci M, Gobbi M, Harris DA, et al. Immunopurification of pathological prion protein aggregates. PLoS One 2009; 4:7816
- Pan T, Li R, Wong BS, Liu T, Gambetti P, Sy MS. Heterogeneity of normal prion protein in two-dimensional immunoblot: presence of various glycosylated and truncated forms. J Neurochem 2002; 81:1092 - 1101
- Beringue V, Mallinson G, Kaisar M, Tayebi M, Sattar Z, Jackson G, et al. Regional heterogeneity of cellular prion protein isoforms in the mouse brain. Brain 2003; 126:2065 - 2073
- Safar JG, DeArmond SJ, Kociuba K, Deering C, Didorenko S, Bouzamondo-Bernstein E, et al. Prion clearance in bigenic mice. J Gen Virol 2005; 86:2913 - 2923
- Barria MA, Mukherjee A, Gonzalez-Romero D, Morales R, Soto C. De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog 2009; 5:1000421
- Westaway D, DeArmond SJ, Cayetano-Canlas J, Groth D, Foster D, Yang SL, et al. Degeneration of skeletal muscle, peripheral nerves and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell 1994; 76:117 - 129
- Chiesa R, Piccardo P, Biasini E, Ghetti B, Harris DA. Aggregated, wild-type prion protein causes neurological dysfunction and synaptic abnormalities. J Neurosci 2008; 28:13258 - 13267
- Fernandez-Funez P, Zhang Y, Casas-Tinto S, Xiao X, Zou WQ, Rincon-Limas DE. Sequence-dependent prion protein misfolding and neurotoxicity. J Biol Chem 2010; 285:36897 - 36908
- Yuan J, Dong Z, Guo JP, McGeehan J, Xiao X, Wang J, et al. Accessibility of a critical prion protein region involved in strain recognition and its implications for the early detection of prions. Cell Mol Life Sci 2008; 65:631 - 643
- Zou WQ, Langeveld J, Xiao X, Chen S, McGeer PL, Yuan J, et al. PrP conformational transitions alter species preference of a PrP-specific antibody. J Biol Chem 2010; 285:13874 - 13884
- Gambetti P, Dong Z, Yuan J, Xiao X, Zheng M, Alshekhlee A, et al. A novel human disease with abnormal prion protein sensitive to protease. Ann Neurol 2008; 63:697 - 708
- Zou WQ, Puoti G, Xiao X, Yuan J, Qing L, Cali I, et al. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann Neurol 2010; 68:162 - 172
- Gambetti P, Puoti G, Zou WQ. Variably Protease-Sensitive Prionopathy: A novel Disease of the Prion Protein. J Mol Neurosci 2011; May 17 [Epub ahead of print]
- Gambetti P, Zou WQ, Torres JM, Soto C, Notari S, Espinosa JC, et al. Variably protease-sensitive prionopathy: Transmissibility and PMCA studies. Prion 2011; 5:14
- Zou WQ, Xiao X, Yuan J, Puoti G, Fujioka H, Wang X, et al. Amyloid-{beta}42 Interacts Mainly with Insoluble Prion Protein in the Alzheimer Brain. J Biol Chem 2011; 286:15095 - 15105
- Chen S, Yadav SP, Surewicz WK. Interaction between human prion protein and amyloid-beta (Abeta) oligomers: role OF N-terminal residues. J Biol Chem 2010; 285:26377 - 26383
- Esiri MM, Carter J, Ironside JW. Prion protein immunoreactivity in brain samples from an unselected autopsy population: findings in 200 consecutive cases. Neuropathol Appl Neurobiol 2000; 26:273 - 284
- Ferrer I, Blanco R, Carmona M, Puig B, Ribera R, Rey MJ, et al. Prion protein expression in senile plaques in Alzheimer's disease. Acta Neuropathol 2001; 101:49 - 56
- Kovacs GG, Zerbi P, Voigtländer T, Strohschneider M, Trabattoni G, Hainfellner JA, et al. The prion protein in human neurodegenerative disorders. Neurosci Lett 2002; 329:269 - 272
- Morales R, Estrada LD, Diaz-Espinoza R, Morales-Scheihing D, Jara MC, Castilla J, et al. Molecular cross talk between misfolded proteins in animal models of Alzheimer's and Prion diseases. J Neurosci 2010; 30:4528 - 4535
- Schwarze-Eicker K, Keyvani K, Görtz N, Westaway D, Sachser N, Paulus W. Prion protein (PrPc) promotes beta-amyloid plaque formation. Neurobiol Aging 2005; 26:1177 - 1182
- Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 2003; 300:636 - 640
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, et al. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell 2003; 115:893 - 904
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell 2003; 115:879 - 891
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell 2010; 140:421 - 435
- Papassotiropoulos A, Wollmer MA, Aguzzi A, Hock C, Nitsch RM, de Quervain DJ. The prion gene is associated with human long-term memory. Hum Mol Genet 2005; 14:2241 - 2246
- Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet 2005; 6:435 - 450
- Tompa P, Friedrich P. Prion proteins as memory molecules: an hypothesis. Neuroscience 1998; 86:1037 - 1043
- Zou WQ. Transmissible spongiform encephalopathy and beyond (E-letter). Science http://www.sciencemag.org/content/308/5727/1420.long/reply#sci_el_10316, 20, Sep 2007