Abstract
The prion diseases sheep scrapie and cervid chronic wasting disease are transmitted, in part, via an environmental reservoir of infectivity; prions released from infected animals persist in the environment and can cause disease years later. Central to controlling disease transmission is the identification of methods capable of inactivating these agents on the landscape. We have found that certain lichens, common, ubiquitous, symbiotic organisms, possess a serine protease capable of degrading prion protein (PrP) from prion-infected animals. The protease functions against a range of prion strains from various hosts and reduces levels of abnormal PrP by at least two logs. We have now tested more than 20 lichen species from several geographical locations and from various taxa and found that approximately half of these species degrade PrP. Critical next steps include examining the effect of lichens on prion infectivity and cloning the protease responsible for PrP degradation. The impact of lichens on prions in the environment remains unknown. We speculate that lichens could have the potential to degrade prions when they are shed from infected animals onto lichens or into environments where lichens are abundant. In addition, lichens are frequently consumed by cervids and many other animals and the effect of dietary lichens on prion disease transmission should also be considered.
Prions in the Environment
Propagation of the transmissible spongiform encephalopathies (TSEs, prion diseases) sheep scrapie and cervid chronic wasting disease (CWD) is dependent upon transfer of infectious scrapie or CWD prions from infected to uninfected hosts. In both TSEs, direct and indirect routes of transmission appear to contribute to disease spread; prions are shed from infected animals and transmission to naïve hosts may result from direct contact with excretions or bodily fluids or from environments contaminated with prions.Citation1,Citation2 Environmental transmission has been known for scrapie for at least 70 years and has only more recently been identified for CWD.Citation3,Citation4 Environmental conditions which inactivate other pathogens are unlikely to affect prion infectivity due to the extreme resistance to degradation of prions.
Soil is a likely fomite for scrapie and CWD transmission that can be contaminated by shed prions, infected carcasses or offal discarded by hunters.Citation5 Deer and other animals commonly consume soil incidentally during feeding or at mineral licks to supplement mineral intake.Citation6 Prions bind to soils and remain infectious and soil-bound prions may actually be more transmissible via the oral route than unbound prions.Citation7,Citation8 Similarly, farm surfaces including gates, fences, posts and troughs retain scrapie prions at least 20 days after depopulating infected animals and exposure to feed buckets, water and bedding from CWD-infected animals was sufficient to cause disease in experimental trials.Citation9,Citation10 While the total duration of prion persistence in the environment is unknown, an observation of environmental scrapie transmission to sheep 16 years after reoccupying a sheep-house previously holding an infected flock, suggests environmental prions may remain infectious for decades.Citation11
Methods of inactivating or degrading TSE agents have long been a focus of research due to concerns about the contamination of foodstuffs, biologicals and medical devices. Evidence demonstrating environmental contamination with TSEs has refocused investigations toward examining environmentally compatible means of inactivating TSE agents. We have recently found that certain lichens may be able to degrade prions due to serine protease activityCitation12 and here, we discuss the potential for lichens to affect TSEs.
The Unique Biology of Lichens
Lichens are unusual organisms composed of a fungus (mycobiont) with at least one symbiotic photosynthetic partner (photobiont).Citation13 Symbiotic algae provide the lichen the ability to fix carbon while cyanobacteria, present in certain species, allow nitrogen and carbon fixation. Among the more than 13,500 species of lichens having been reported, only about 100 photobionts have been identified, reflecting use of identical photobionts by distinct mycobionts.Citation14 It is probable that all photobionts can live freely, but mycobionts are dependent on symbiosis with photobionts for survival in nature. Along with photobionts, lichens can also be home to communities of bacteria, which may also contribute to the survival of the entire organism.Citation15
Lichens are evolutionarily successful and the lichen symbiosis has independently evolved multiple times.Citation14 Carbon, and often, nitrogen fixation capabilities allow lichens to be self-sufficient and able to inhabit diverse ecosystems ranging from arctic tundra to equatorial deserts.Citation13 Tree trunks and branches, vegetation, soil, bare rock and human-made objects are frequently colonized by lichens. The abundance of lichens in different ecosystems is variable and ranges from only a small fraction of the total biomass, to lichens being a predominant organism on the landscape. Approximately 8% of the land surface on Earth is dominated by lichens.Citation16 Four ecosystems in which lichens can be the predominant vegetative species are depicted in ; in tundra (A), boreal forests (B), deciduous forest breaks (C) or desert crusts (D), lichens are abundant as ground cover. Lichens can also cover large amounts of surface area when growing on other vegetation as epiphytes ( and inset).
A characteristic of lichens is the production of unusual secondary metabolites, some of which are not known to occur elsewhere in nature.Citation17 Quantities of these compounds in lichens can reach nearly 50% of dry weight and some can be found in soil or other environments adjacent to lichens due to leaching.Citation18 Secondary metabolites produced by lichens can aid in their survival by protecting against ultraviolet radiation and desiccation, deterring herbivores and impeding growth of competitors, such as vascular plants or microbes.Citation17 This latter property has made lichens the focus of herbicide and antibiotic discovery efforts. The presence of unique biomolecules and the widespread and abundant geographic distribution of lichens motivated us to explore whether these organisms or their extracts could affect prion protein (PrP) in a series of in vitro experiments.
Degradation of Prion Protein by a Lichen Protease
As an initial effort to examine the effect of lichens on PrP, we prepared organic extracts from various archived or freshly collected lichen species using a method known to extract secondary metabolites and other molecules from lichens.Citation12 We exposed PrP-enriched preparations made from TSE-infected brains to the lichen extracts and examined PrP levels after incubation. We found that three of the extracts, from Parmelia sulcata, Cladonia rangiferina and Lobaria pulmonaria, were able to reduce prion protein levels below the limit of immunblotting detection, indicating at least two logs of reduction. These lichen extracts also reduced PrP levels in brain homogenates from hamsters clinically-positive with HY, DY or 263K strains of TSE, mice clinically-positive with RML strain and CWD-positive deer. We quantified reductions using protein misfolding cyclic amplification (PMCA) to measure the amount of prion seeding activity remaining in lichen extract-treated samples. We minimally found a reduction of two logs of PMCA activity following lichen extract treatment, in agreement with our immunoblotting results. Both cellular PrP and an unrelated protein, protein C5 of the 20S proteosome, were also susceptible to proteolysis by lichen extracts, however we have not completely characterized the specificity of lichen proteases.
Activity of lichens to reduce PrP levels was mediated by a serine protease, as the specific inhibitor Pefabloc SC was able to block PrP degradation and inhibitors of other classes of enzymes or proteases were not effective. We also tested the hypothesis that common lichen secondary metabolites, such as atranorin, usnic acid and lecanoric acid, would affect PrP levels, but found no effect of these compounds, even at high concentrations. Lichen anti-PrP activity was extremely species-specific; lichen species in the same genera as those with activity did not necessarily also possess activity. We tested extracts of isolated lichen photobionts, but did not observe any anti-PrP activity, suggesting that the fungal component of lichen was responsible for activity.
Since our initial observations of PrP degradation by lichen extracts, we have tested >20 species of lichens from at least 13 genera collected at various geographical locations. The results from these studies are presented in . We have found organic extracts of about half of the tested species can reduce PrP levels. Identical lichens collected at different geographical locations had similar levels of activity, supporting the concept of species specificity in PrP degradation. Not all of our data, however, suggest that lichen serine proteases all function identically. We found that P. sulcata and C. rangiferina extracts were active at pH 4.0 and had reduced activity at neutral or elevated pH. Extract from L. pulmonaria had similar activity independent of pH, suggesting structural or mechanistic differences in the L. pulmonaria serine protease.
The identification that lichens possess an anti-PrP protease activity may prove useful for expanding the repertoire of methods for degrading prions. We were also interested if lichen could promote PrP degradation under more natural conditions. To more closely simulate environmental conditions, we exposed PrP-enriched preparations or infected brain homogenate to either a small quantity of intact P. sulcata tissue or an aqueous extract of the lichen. We found that both lichen tissue and aqueous extract were able to reduce PrP levels, suggesting lichens have the potential to degrade PrP in the environment.
Comparison with Other Serine Proteases
Many studies have been performed to test the susceptibility of PrP to proteolysis and serine proteases recurrently appear to be among the most active in degrading PrP.Citation19–Citation24 Serine proteases are characterized by the presence of a serine group at the center of their active site and one of the most common serine proteases, proteinase K (PK), is widely used to test for the presence of abnormal PrP. Both others and we have found PK, even at high concentrations, has limited activity to degrade abnormal PrP.Citation12,Citation25 Other serine proteases including subtilisins, the bacterial proteinase “prionase”, Streptomyces E77 protease and PWD-1 keratinase have all shown great promise in degrading PrP,Citation19–Citation24 even when bound to soil.Citation26 Typical conditions used for prion inactivation by proteases, however, involve elevated temperatures, the presence of detergents and extreme pH values. The serine protease activity that we have identified in lichens functions at ambient or physiological temperature, in the absence of detergents and at low or neutral pH. A clear and necessary next step is sequencing the lichen protease for comparison with other proteases.
Sequencing efforts are underway, but may prove complicated due to the multiorganism nature of lichens and incomplete information regarding whether proteases are produced by mycobionts, photobionts or lichen-associated bacteria. Few lichen mycobionts can be cultured in the absence of photobionts and gene expression in each organism is almost certainly changed upon establishment of lichen symbiosis.Citation27 Efforts to sequence the genomes of organisms composing lichens will undoubtedly assist in sequencing lichen proteases capable of degrading PrP.Citation28 Another complication to understanding biological activities in lichens is that proteins produced by one organism may be subject to post-translational modifications by the other organism(s) present in the symbiosis. Much evidence exists for post-translational modification of proteases in other biological systemsCitation29 and these processes may affect the PrP-proteolytic activity of lichens. Additionally, lichen secondary metabolites, co-enzymes and other cofactors may also contribute to PrP degradation by activating lichen proteases or sensitizing PrP to proteolysis.
A Role for Lichens in Controlling TSEs on the Landscape?
The concept that lichens may be useful in controlling TSEs is intriguing and, with much caution, in this section we will begin to speculate about how lichens could limit TSE transmission on the landscape. The potential is greatest for lichens to affect CWD transmission as, in contrast with TSEs affecting domestic species, prions are released into environments where lichens can be abundant and free-ranging cervids consume lichens as food. Presently, our data about the effects of lichens on TSEs are limited, but do indicate that lichens affect two common surrogate markers for TSE infectivity. Namely, lichen organic and aqueous extracts can degrade PK-resistant PrP and lichen organic extracts cause reductions in PMCA templating activity. Levels of PrP, however, often fail to completely correlate with infectious TSE titer and investigation into the effect of lichens and their extracts on infectivity is needed and ongoing.
Should lichens be able to inactivate or degrade TSE infectivity, both indirect and direct modes of CWD transmission could be affected (). Prions are shed from infected animals in secretions, excretions or from infected carcasses and enter the environment where they persist in soil or on other fomite surfaces and transmit disease to naïve hosts.Citation5 Lichens possess no external cuticle or epidermis to limit uptake of substances into the lichen.Citation13 Should CWD prions be deposited onto lichens, proteases and prions may easily come into direct contact and protease activity may be able to degrade the prion infectivity. Similarly, the lack of cuticle or epidermis in lichens allows leaching of lichen substances into the environment.Citation18 As we have found that aqueous extracts of lichens can degrade PrP, lichens growing on or near contaminated fomites may leach proteases capable of degrading prions on these surfaces. The activity of lichen proteases, especially when leached from lichens, toward surface-bound PrP is unknown and an important topic of future research.
The consumption of lichens by wild-life species, and especially cervids, is well known. For example, in arctic climates lichens minimally constitute 60% of the winter diet of caribou.Citation30 While the diets of cervids in other climates are highly variable, lichens are desirable, and in some cases preferred, browse.Citation31,Citation32 The effect of lichen consumption on CWD transmission is unknown, but should lichen proteases promote degradation of CWD prions in the gastrointestinal tract, lichen consumption could affect both direct and indirect transmission of disease by reducing the infectious dose to which the host is exposed. It is unclear if lichen proteases would remain active following consumption. Endogenous protease inhibitors secreted by the host may inactivate lichen proteolytic activity in the digestive tract. Similarly, gastrointestinal and rumen microbes, low gastric pH and digestive enzymes all contribute to the breakdown of ingested protein and may degrade lichen proteases prior their contact with prions. Limited evidence, however, indicates poor protein bioavailability in ruminants fed on lichens,Citation33 suggesting protease activity might be preserved in distal portions of the digestive system. Clearly, further experimental trials are needed to assess what effect, if any, lichens may have on CWD transmission. Our results suggest, however, that the effects of lichens on TSEs are worth consideration.
Abbreviations
| CWD | = | Chronic Wasting disease |
| PK | = | proteinase K |
| PrP | = | prion protein |
| PMCA | = | protein misfolding cyclic amplification |
| TSE | = | transmissible spongiform encephalopathy |
Figures and Tables
Figure 1 Lichens can be a predominant ground cover in various, diverse environments. (A) Lichens are one of the few types of vegetation capable of surviving in tundra and are found covering soil. Photographer: Daniel Ruthrauff (USGS). (B) In boreal forests, lichens form thick mats covering the soil. Photographer: Cephas. (C) Lichens in deciduous forest breaks can be found in abundance as ground cover, as seen on the mine tailings in the photo, or growing on trees as epiphytes (inset). Photographer: James Bennett (USGS). (D) Biological soil crusts (dark surfaces) form on desert soils and are composed of a community of organisms, including lichens. Photographer: Charles Schelz (National Park Service).
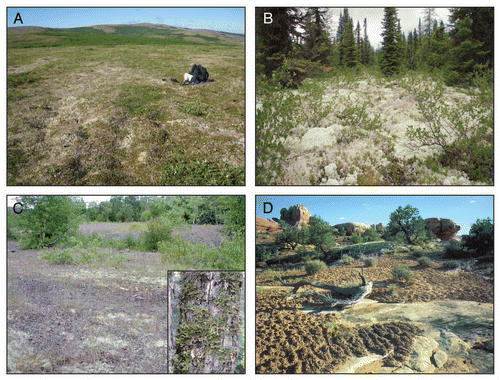
Figure 2 Hypothetical points where lichens could interrupt CWD transmission cycles. Transmission of CWD occurs directly through animal-to-animal contact and through indirect routes of exposure. Soil and other fomites are thought to maintain CWD infectivity in the environment and cause disease following oral exposure to prions. Should CWD agent be shed from infected animals or released from infected carcasses onto lichen surfaces, protease activity from the lichens may degrade the prions. Lichen leachates containing protease activity may also be able to degrade prions bound to soil or other fomite surfaces. The consumption of lichens by healthy animals may block both direct and indirect CWD transmission by promoting degradation of prion in the gastrointestinal tract.
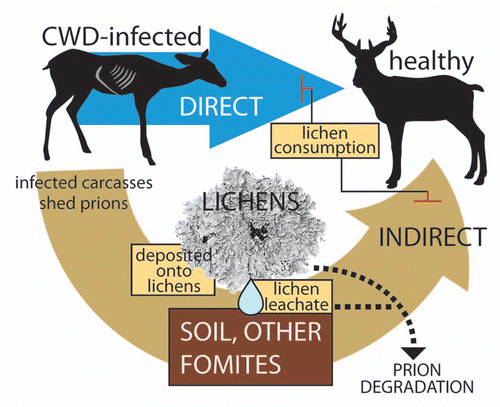
Table 1 Activity of various lichens to degrade PrP from infected hamsters (HY strain)
Acknowledgments
This work was funded by USGS Wildlife: Terrestrial and Endangered Resources Program. We thank Dr. Dennis Heisey, Dr. Christopher Brand, Christina Carlson and Nicole Gibbs for their valuable comments and the photographers whom we have listed for the use of their images. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- Detwiler LA, Baylis M. The epidemiology of scrapie. Rev Sci Tech 2003; 22:121 - 143
- Sigurdson CJ. A prion disease of cervids: Chronic wasting disease. Vet Res 2008; 39:41
- Greig JR. Scrapie: Observations on the transmission of the disease by mediate contact. Vet J 1940; 96:203 - 206
- Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 2004; 10:1003 - 1006
- Schramm PT, Johnson CJ, McKenzie D, Aiken JM, Pedersen JA. Potential role of soil in the transmission of prion disease. Rev Mineral Geochem 2006; 64:125 - 132
- Mahaney WC, Krishnamani R. Understanding geophagy in animals: Standard procedures for sampling soils. J Chem Ecol 2003; 29:1503 - 1522
- Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog 2006; 2:32
- Johnson CJ, Pedersen JA, Chappell R, McKenzie D, Aiken JM. Oral transmissibility of prions is enhanced by binding to soil particles. PLoS Pathog 2007; 3:93
- Maddison BC, Baker CA, Terry LA, Bellworthy SJ, Thorne L, Rees HC, et al. Environmental sources of scrapie prions. J Virol 2010; 84:11560 - 11562
- Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, et al. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS ONE 2009; 4:5916
- Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol 2006; 87:3737 - 3740
- Johnson CJ, Bennett JP, Biro SM, Duque-Velasquez JC, Rodriguez CM, Bessen RA, et al. Degradation of the disease-associated prion protein by a serine protease from lichens. PLoS ONE 2011; 6:19836
- Purvis W. Lichens 2000; Washington DC Smithsonian Books
- Lutzoni F, Miadlikowska J. Lichens. Curr Biol 2009; 19:502 - 503
- Bates ST, Cropsey GW, Caporaso JG, Knight R, Fierer N. Bacterial communities associated with the lichen symbiosis. Appl Environ Microbiol 2011; 77:1309 - 1314
- Larson DW. Peveling E. The absorption and release of water by lichens. Bibliothea Lichenologia: Progress and Problems in Lichenology in the Eighties 1987; Berlin-Stuttgart J Cramer 351 - 360
- Huneck S, Yoshimura I. Identification of Lichen Substances 1996; Berlin & New York Springer
- Dawson HJ, Hrutfiord BF, Ugolini FC. Mobility of lichen compounds from Cladonia mitis in arctic soils. Soil Sci 1984; 138:40 - 45
- Pilon JL, Nash PB, Arver T, Hoglund D, Vercauteren KC. Feasibility of infectious prion digestion using mild conditions and commercial subtilisin. J Virol Methods 2009; 161:168 - 172
- McLeod AH, Murdoch H, Dickinson J, Dennis MJ, Hall GA, Buswell CM, et al. Proteolytic inactivation of the bovine spongiform encephalopathy agent. Biochem Biophys Res Commun 2004; 317:1165 - 1170
- Langeveld JP, Wang JJ, Van de Wiel DF, Shih GC, Garssen GJ, Bossers A, et al. Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis 2003; 188:1782 - 1789
- Hui Z, Doi H, Kanouchi H, Matsuura Y, Mohri S, Nonomura Y, et al. Alkaline serine protease produced by Streptomyces sp. degrades PrP(sc). Biochem Biophys Res Commun 2004; 321:45 - 50
- Lawson VA, Stewart JD, Masters CL. Enzymatic detergent treatment protocol that reduces protease-resistant prion protein load and infectivity from surgical-steel monofilaments contaminated with a human-derived prion strain. J Gen Virol 2007; 88:2905 - 2914
- Yoshioka M, Murayama Y, Miwa T, Miura K, Takata M, Yokoyama T, et al. Assessment of prion inactivation by combined use of Bacillus-derived protease and SDS. Biosci Biotechnol Biochem 2007; 71:2565 - 2568
- Meade-White KD, Barbian KD, Race B, Favara C, Gardner D, Taubner L, et al. Characteristics of 263K scrapie agent in multiple hamster species. Emerg Infect Dis 2009; 15:207 - 215
- Saunders SE, Bartz JC, Vercauteren KC, Bartelt-Hunt SL. Enzymatic digestion of chronic wasting disease prions bound to soil. Environ Sci Technol 2010; 44:4129 - 4135
- Joneson S, Armaleo D, Lutzoni F. Fungal and algal gene expression in early developmental stages of lichen-symbiosis. Mycologia 2011; 103:291 - 306
- Armaleo D, May S. Sizing the fungal and algal genomes of the lichen Cladonia grayi through quantitative PCR. Symbiosis 2009; 49:43 - 51
- Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew Chem Int Ed Engl 2005; 44:7342 - 7372
- Thomas DC, Edmonds JE, Brown WK. The diet of woodland caribou populations in west-central Alberta. Rangifer 1994; 9:337 - 342
- Ward RL, Marcum CL. Lichen litterfall consumption by wintering deer and elk in western Montana. J Range Manage 2005; 69:1081 - 1089
- Hodgman TP, Bowyer RT. Winter use of arboreal lichens, Ascomycetes, by white-tailed deer, Odocoileus virginianus, in Maine. Canadian Field-Naturalist 1985; 99:313 - 316
- Robbins CT. Digestability of an arboreal lichen by mule deer. J Range Manage 1987; 40:491 - 492