Abstract
Protein phosphatase 5 plays a pivotal role in signal transduction in animal and plant cells, and it was previously shown that Arabidopsis protein phosphatase 5 (AtPP5) performs multiple enzymatic activities that are mediated by conformational changes induced by heat shock stress. In addition, transgenic overexpression of AtPP5 gene conferred enhanced heat shock resistance compared with wild-type plant. However, the molecular mechanism underlying this enhanced heat shock tolerance through functional and conformational changes upon heat stress is not clear. In this report, AtPP5 was shown to preferentially interact with its substrate, MDH, under heat stress conditions. In addition, in co-IP analysis, AtPP5 was observed to form a complex with AtHsp90 in Arabidopsis. These results suggest that AtPP5 may enhance thermotolerance via forming multi-chaperone complexes under heat shock conditions in Arabidopsis. Finally, we show that AtPP5 is primarily localized in the cytoplasm of Arabidopsis.
The tetratricopeptide repeat (TPR) was identified and named in 1990, and its name denotes a 34 amino acid sequence that comprises a repeating structural unit.Citation1,Citation2 The first TPR crystal structure was solved using the three-TPR domain of protein phosphatase 5 (PP5). Recently, nuclear localized AtPP5 was shown to play as a biologically active photoreceptor.Citation3 However, the principal and functional roles of cytoplasmic AtPP5 are largely unknown. In previous reports, many TPR-containing proteins have been shown to exhibit molecular chaperone functions.Citation4-Citation9 However, PP5 has not been shown to display chaperone function in vitro or in vivo. Recently, we have reported that AtPP5 demonstrated protein phosphatase activity and also protein chaperon function as a TPR-containing protein.Citation10 The AtPP5 chaperone function acts as a biological drive for survival, increasing its thermotolerance for protein aggregation protection and facilitating the refolding of damaged proteins resulting from heat shock stress.
Heat Shock Treatment Confers Physical Interaction between AtPP5 and its Substrate MDH
Hsp90-associated proteins, FKBP52, p23, and CHIP, possess intrinsic chaperone activities that enable them to recognize and bind nonnative proteins.Citation11-Citation14 These proteins are also known to possess TPR structural domains that participate in protein-protein interactions.Citation4-Citation6 TPR-containing AtPP5 has been shown to form a complex with Hsp90 and has properties similar to FKBP52 and FKBP51 in mammalian cells.Citation15 In addition, Hsp90-associated TPR-containing proteins exhibit a holdase chaperone activity. This study reported here sought to determine whether AtPP5 binds to partially denatured proteins, as some molecular chaperones have been reported to physically associate with denatured substrates to inhibit non-productive aggregation. SEC analysis showed that AtPP5 did not interact with substrate protein MDH at an incubation temperature of 22°C (), possibly because at this temperature MDH remains in its native conformation. However, when AtPP5 was incubated with MDH at 42°C for 20 min, a significant amount of MDH was co-eluted with the high molecular weight (HMW) form of AtPP5. Physical association of AtPP5 with nonnative MDH was verified by SDS-PAGE, which showed that AtPP5 and MDH subunits were present in the SEC HMW fraction (). This protein interaction was associated with a concomitant decrease in the amount of free MDH (). We have further investigated the interaction of AtPP5 with MDH under heat shock conditions by using co-immunoprecipitation (co-IP) techniques (). Under normal conditions at 22°C, AtPP5 did not interact with MDH. However, under heat stress conditions of 42°C for 20 min, AtPP5 was shown to interact with MDH. These results suggest that AtPP5 preferentially interacts with heat-denatured MDH.
Figure 1. Physical association of AtPP5 with MDH by FPLC and Co-IP analysis. After pre-incubating 20 µM AtPP5 with 10 µM MDH for 20 min at either (A) 22°C or (B) 42°C, the mixtures were separated by SEC (upper panels of A and B). Each fraction was analyzed on SDS-PAGE followed by silver staining (bottom panels of A and B). (C) In vitro co-IP of AtPP5 and MDH at 22°C and 42°C. The presence of MDH was detected on western blot using an anti-MDH antibody. The input lane was loaded with 2% of the reaction mixture. The arrow (→) indicates the IgG large subunit.
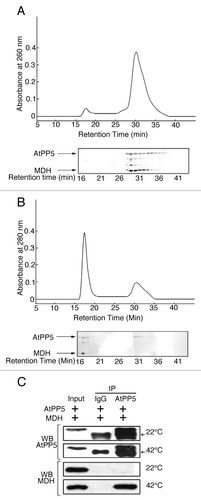
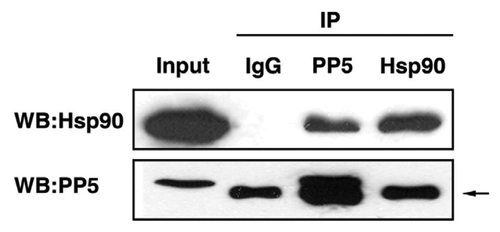
Figure 2. Interaction of AtPP5 and AtHsp90 by co-IP. In vivo co-IP of AtPP5 and AtHsp90. Co-IP of (lane 3) AtHsp90 with antiserum against AtPP5 and (lane 4) AtPP5 with antiserum against AtHsp90, followed by western blotting and detection with anti-AtHsp90 antibody and anti-AtPP5 antibody, respectively. The arrow (→) indicates the IgG large subunit.
Multiple Chaperone Complex of AtPP5 with AtHsp90
To screen for proteins interacting with AtPP5 in plant cells, co-IP was performed using protein extract from Arabidopsis suspension culture cells. Interacting proteins isolated by AtPP5 co-IP were identified by 2-D PAGE followed by MALDI-TOF analyses. One of the isolated, putative AtPP5-interacting proteins was identified as cytosolic heat shock protein 90.2 (AtHsp90.2), which has been shown to interact with PP5 in mammalian cells.Citation16 TPR domain-containing proteins involved in the maturation of HSP90 complexes have been well characterized.Citation17 Members of the HSP90 protein family proteins are found to be associated with inactive or unstable protein substrates inside the cell. Thus, they act to prevent aggregation and/or permit rapid activation of their respective substrates.Citation18,Citation19 PP5 has also been observed to form a complex with Hsp90 and has properties similar to those of mammalian FKBP52 and FKBP51.Citation15 In addition, PP5 has been shown to bind with HSP90 in tomato, and the resultant protein complex is involved in disease response signaling.Citation20
To confirm the interaction between AtPP5 and AtHsp90.2 in vitro, co-IP was performed. Intact AtHsp90.2 or AtPP5 was readily expressed and purified from E. coli. Purified anti-AtPP5 or anti-AtHsp90.2 antibody was incubated with recombinant AtPP5 and/or AtHsp90.2, respectively. To determine the antibody specificity, SDS-PAGE and western blot analyses using an anti-AtPP5 antibody or anti-AtHsp90.2 antibody were performed with an Arabidopsis suspension culture cell extract. Both AtPP5 and AtHsp90.2 were specifically co-immunoprecipitated by anti-AtPP5 antibody but not by pre-immune serum (). These results confirmed the in vitro interaction of AtPP5 with AtHsp90 in Arabidopsis suspension culture cell extract. Hydrophobic interaction between a chaperone and its substrate is a major force that drives the association and binding of these proteins.Citation21 The functional difference between AtPP5 and AtHsp90.2 seemed to be derived from differences in their structure and hydrophobicity. These results suggest that AtPP5 and AtHsp90.2 may form multi-chaperone complexes under heat shock conditions in Arabidopsis.
Subcellular Localization of GFP-tagged AtPP5 Proteins
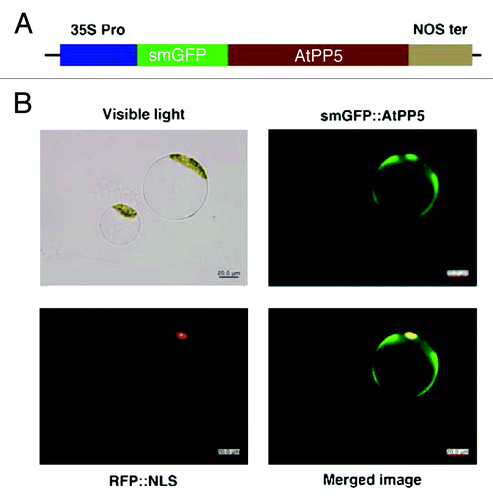
It has been reported that PP5 is predominately distributed in the cytoplasm and nucleus of mammalian cells.Citation22 However, the localization pattern of PP5 in Arabidopsis is largely unknown. Recently, it was shown that AtPP5 is localized in the nucleus and it acts on biologically active phytochromes.Citation3 To examine the subcellular distribution of AtPP5 and determine whether its localization is responsible for its multiple functions, we constructed a chimeric protein comprising a soluble, modified green fluorescent protein (smGFP) fused to full-length AtPP5. smGFP was fused in-frame to the N-terminus of AtPP5 (). The resulting construct, smGFP::AtPP5, was transiently expressed in Arabidopsis protoplasts. Using smGFP as a reporter, we identified the intracellular localization of AtPP5 in Arabidopsis. Imaging of the smGFP fluorescence in cells by confocal laser scanning microscopy showed that AtPP5 is primarily localized in the cytoplasm ().
Figure 3. Subcellular localization of AtPP5 fused with GFP in Arabidopsis protoplasts. (A) Schematic diagram of plasmid construct for transforming plant cells for fluorescent confocal microscopy. Expression of the fused genes was driven by the CaMV 35S promoter (35S Pro) and terminated by the nopaline synthetase terminator (NOS ter). Arabidopsis protoplasts were transformed with the resultant constructs and fluorescent images were obtained 12 to 48 h after transformation. Green and red images are GFP and RFP fluorescence signals of smGFP::AtPP5 and RFP::NLS, respectively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by RDA for the Next-Generation BioGreen Program (SSAC, No. 2011), by the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ007850), and by MOEST for the WCU program (R32–10148). H.B.C. was supported by the BK21 Program, Korea.
References
- Hirano T, Kinoshita N, Morikawa K, Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell 1990; 60:319 - 28; http://dx.doi.org/10.1016/0092-8674(90)90746-2; PMID: 2297790
- Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell 1990; 60:307 - 17; http://dx.doi.org/10.1016/0092-8674(90)90745-Z; PMID: 2404612
- Ryu JS, Kim JI, Kunkel T, Kim BC, Cho DS, Hong SH, et al. Phytochrome-specific type 5 phosphatase controls light signal flux by enhancing phytochrome stability and affinity for a signal transducer. Cell 2005; 120:395 - 406; http://dx.doi.org/10.1016/j.cell.2004.12.019; PMID: 15707897
- Bose S, Weikl T, Bügl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science 1996; 274:1715 - 7; http://dx.doi.org/10.1126/science.274.5293.1715; PMID: 8939863
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science 1996; 274:1718 - 20; http://dx.doi.org/10.1126/science.274.5293.1718; PMID: 8939864
- Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol 2001; 308:795 - 806; http://dx.doi.org/10.1006/jmbi.2001.4595; PMID: 11350175
- Yano M, Terada K, Mori M. Mitochondrial import receptors Tom20 and Tom22 have chaperone-like activity. J Biol Chem 2004; 279:10808 - 13; http://dx.doi.org/10.1074/jbc.M311710200; PMID: 14699115
- Rosser MF, Washburn E, Muchowski PJ, Patterson C, Cyr DM. Chaperone functions of the E3 ubiquitin ligase CHIP. J Biol Chem 2007; 282:22267 - 77; http://dx.doi.org/10.1074/jbc.M700513200; PMID: 17545168
- Zabka M, Lesániak W, Prus W, Kuzánicki J, Filipek A. Sgt1 has co-chaperone properties and is up-regulated by heat shock. Biochem Biophys Res Commun 2008; 370:179 - 83; http://dx.doi.org/10.1016/j.bbrc.2008.03.055; PMID: 18358234
- Park JH, Lee SY, Kim WY, Jung YJ, Chae HB, Jung HS, Kang CH, Shin MR, Kim SY, Su'udi M, Yun DJ, Lee KO, Kim MG. Heat-induced chaperone activity of serine/threonine protein phosphatase 5 enhances thermotolerance in Arabidopsis thaliana. New Phytol.
- Hildenbrand ZL, Molugu SK, Herrera N, Ramirez C, Xiao C, Bernal RA. Hsp90 can accommodate the simultaneous binding of the FKBP52 and HOP proteins. Oncotarget 2011; 2:43 - 58; PMID: 21378414
- Karagöz GE, Duarte AM, Ippel H, Uetrecht C, Sinnige T, van Rosmalen M, et al. N-terminal domain of human Hsp90 triggers binding to the cochaperone p23. Proc Natl Acad Sci U S A 2011; 108:580 - 5; http://dx.doi.org/10.1073/pnas.1011867108; PMID: 21183720
- Bento CF, Fernandes R, Ramalho J, Marques C, Shang F, Taylor A, et al. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS One 2010; 5:e15062; http://dx.doi.org/10.1371/journal.pone.0015062; PMID: 21124777
- Rao SN, Sharma J, Maity R, Jana NR. Co-chaperone CHIP stabilizes aggregate-prone malin, a ubiquitin ligase mutated in Lafora disease. J Biol Chem 2010; 285:1404 - 13; http://dx.doi.org/10.1074/jbc.M109.006312; PMID: 19892702
- Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem 1997; 272:16224 - 30; http://dx.doi.org/10.1074/jbc.272.26.16224; PMID: 9195923
- Skarga Y, Vrublevskaya V, Evdokimovskaya Y, Morenkov O. Purification of the 90 kDa heat shock protein (hsp90) and simultaneous purification of hsp70/hsc70, hsp90 and hsp96 from mammalian tissues and cells using thiophilic interaction chromatography. Biomed Chromatogr 2009; 23:1208 - 16; http://dx.doi.org/10.1002/bmc.1245; PMID: 19488974
- Zhao JM, Liu T, Tian L, Wei YQ, Wen YJ. [Construction of mammalian co-expression plasmid pIRES -LMP1-HSP90 beta and its expression in vitro]. Sichuan Da Xue Xue Bao Yi Xue Ban 2006; 37:339 - 43; PMID: 16761403
- Pearl LH, Prodromou C. Structure, function, and mechanism of the Hsp90 molecular chaperone. Adv Protein Chem 2001; 59:157 - 86; http://dx.doi.org/10.1016/S0065-3233(01)59005-1; PMID: 11868271
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003; 228:111 - 33; PMID: 12563018
- de la Fuente van Bentem S, Vossen JH, de Vries KJ, van Wees S, Tameling WI, Dekker HL, et al. Heat shock protein 90 and its co-chaperone protein phosphatase 5 interact with distinct regions of the tomato I-2 disease resistance protein. Plant J 2005; 43:284 - 98; http://dx.doi.org/10.1111/j.1365-313X.2005.02450.x; PMID: 15998314
- Klappa P, Hawkins HC, Freedman RB. Interactions between protein disulphide isomerase and peptides. Eur J Biochem 1997; 248:37 - 42; http://dx.doi.org/10.1111/j.1432-1033.1997.t01-1-00037.x; PMID: 9310357
- de la Fuente van Bentem S, Vossen JH, Vermeer JE, de Vroomen MJ, Gadella TW Jr., Haring MA, et al. The subcellular localization of plant protein phosphatase 5 isoforms is determined by alternative splicing. Plant Physiol 2003; 133:702 - 12; http://dx.doi.org/10.1104/pp.103.026617; PMID: 12972652