Abstract
Vesicle fusion processes in plants are important for both development and stress responses. Transgenic potato plants with reduced expression of SYNTAXIN-RELATED1 (StSYR1), a gene encoding the potato homolog of Arabidopsis PENETRATION1 (AtPEN1), display spontaneous necrosis and chlorosis at later stages of development. In accordance with this developmental defect, tuber number, weight and overall yield are significantly reduced in StSYR1-RNAi lines. Enhanced resistance of StSYR1-RNAi plants to Phytophthora infestans, the causal agent of late blight disease of potato, correlates with enhanced levels of salicylic acid, whereas levels of 12-oxophytodienoic acid and jasmonic acid are unaltered. Cultured cells of StSYR1-RNAi lines secrete at least two compounds which are not detectable in the supernatant of control cells, suggesting an involvement of StSYR1 in secretion processes to the apoplast.
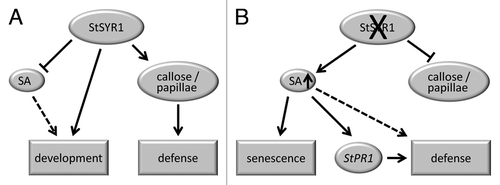
The secretory system of plants is involved in diverse fundamental aspects of development and adaptation to various environmental conditions. SNAREs (SOLUBLE N-ETHYLMALEIMIDE SENSITIVE FACTOR ATTACHMENT PROTEIN RECEPTOR) are membrane-resident proteins mediating selective fusion of transport vesicles with the membrane of the target compartment for delivery of their cargo. We earlier described an RNA-interference approach to functionally analyze the SNARE-protein StSYR1 (SYNTAXIN-RELATED 1) in potato (Solanum tuberosum cv Désirée). StSYR1-RNAi plants displayed highly reduced expression of the close homologs StSYR1–1 and StSYR1–2, which share 85% homology at the protein level.Citation1 Homologs of StSYR1 had been functionally characterized in tobacco (NtSYR1),Citation2 barley (HvROR2) and Arabidopsis (AtPEN1 = AtSYP121).Citation3 The latter two were shown to be important for penetration resistance against Blumeria graminis f.sp hordei (Bgh).Citation3 In potato StSYR1-RNAi plants, developmental defects as well as altered defense responses were observed. The results obtained by the functional analyses of these plants are summarized in the model depicted in . In detail, in wild type potato plants, StSYR1 functions in development, one mechanism being the negative regulation of SA levels, as was described for the Arabidopsis homolog AtPEN1.Citation4 In response to infection with Phytophthora infestans, StSYR1 is required for proper deposition of callose-containing papillae (). In StSYR1-RNAi plants, the inhibitory effect of StSYR1 on SA accumulation is abolished, resulting in constitutively enhanced SA levels and, concomitantly, an early senescence phenotype, which is known to be related to high SA contents.Citation5 The elevated SA levels correlate with enhanced StPR1 gene expression and enhanced defense responses against P. infestans. Moreover, StSYR1-RNAi plants are unable to form normal callose-containing papillae, suggesting that StSYR1 is required for the delivery of cell wall components to the site of penetration ().
Developmental Defects of StSYR1-RNAi Plants
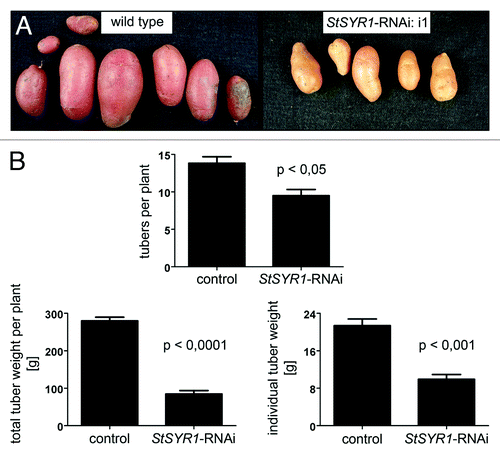
Under greenhouse conditions, StSYR1-RNAi plants are impaired in tuber development. As shown in , representative tubers of control plants develop a red color, specific to cv Désirée. The coloring of tubers of StSYR1-RNAi plants, however, is less pronounced, suggesting an effect on anthocyanine accumulation. Additionally, RNAi plants produce fewer tubers with reduced weight, resulting in a reduction of the overall yield (). These are most likely secondary effects due to the impaired growth and development, emphasizing the role of StSYR1 for normal development.
Figure 2.StSYR1-RNAi lines are impaired in tuber development. Plants were cultivated in the green house in five liter pots under standard conditions. After about three months, tubers had matured and were harvested. (A) Representative tubers of wild type plants and one StSYR1-RNAi line (i1) are shown. (B) The amounts of tubers per plant, as well as the tuber weights, totally per plant and individually are plotted. “Control,” wild type and empty vector-transformed plants; “StSYR1-RNAi,” 11 independent StSYR1-RNAi construct-transformed lines. Statistically significant differences are depicted (GraphPad Prism 5, Mann-Whitney-Test). The experiment was performed three times with similar results.
Jasmonic Acid Levels are not Altered in StSYR1-RNAi Plants
Both SA and jasmonic acid (JA) are known to be involved in plant defense signaling. Although in Arabidopsis and other plants, antagonistic effects of both phytohormones had been demonstrated, more complex regulation mechanisms were described as well.Citation6 In potato, SA, but not JA is important for the basal resistance against infection with P. infestans.Citation7,Citation8 On the other hand, both compounds are required for the activation of PAMP-induced defense responses in potato, with SA acting upstream of JA, since (1) plants silenced in the JA biosynthesis pathway are still able to accumulate SA in response to infiltration of Pep-13, a Phytophthora PAMP (pathogen-associated molecular pattern)Citation9 and, (2) plants depleted of SA due to the expression of the NahG gene (salicylate hydroxylase), fail to accumulate JA.Citation8,Citation10 In this context, it was interesting to determine possible effects of the elevated SA levels in StSYR1-RNAi lines on endogenous JA accumulation. Leaf material of plants grown under controlled conditions in a phytochamber was harvested and subjected to JA-measurements.Citation11 For JA, its biosynthetic intermediate 12-oxo-phytodienoic acid (OPDA) and the JA derivative JA-isoleucine (JA-Ile), no statistically significant differences to control plants were measured (). Thus, increased SA-levels in StSYR1-RNAi plants do not have an effect on the accumulation of JA.
Figure 3. Accumulation of jasmonates is not altered in StSYR1-RNAi plants. Plants were grown for three weeks in a phytochamber with 16 h light (140 µE) at 60% humidity and 20°C. Leaf material of healthy plants was sampled and used for the extraction of jasmonates. Concentrations of 12-oxo-phytodienoic acid [OPDA; (A), JA (B) and JA-isoleucine (JA-Ile; (C)] are plotted. Control, wild type and empty vector-transformed plants; StSYR1-RNAi, StSYR1-RNAi construct-transformed lines; fw, fresh weight. The experiment was done twice with similar results with at least ten independent StSYR1-RNAi lines. No statistically significant differences between control and StSYR1-RNAi lines were measured (ns, not significant; GraphPad Prism 5, Mann-Whitney-Test).
![Figure 3. Accumulation of jasmonates is not altered in StSYR1-RNAi plants. Plants were grown for three weeks in a phytochamber with 16 h light (140 µE) at 60% humidity and 20°C. Leaf material of healthy plants was sampled and used for the extraction of jasmonates. Concentrations of 12-oxo-phytodienoic acid [OPDA; (A), JA (B) and JA-isoleucine (JA-Ile; (C)] are plotted. Control, wild type and empty vector-transformed plants; StSYR1-RNAi, StSYR1-RNAi construct-transformed lines; fw, fresh weight. The experiment was done twice with similar results with at least ten independent StSYR1-RNAi lines. No statistically significant differences between control and StSYR1-RNAi lines were measured (ns, not significant; GraphPad Prism 5, Mann-Whitney-Test).](/cms/asset/e6f6f06f-fa16-4801-9a10-2a516139e477/kpsb_a_10919866_f0003.gif)
Metabolites Secreted by Cultured StSYR1-RNAi Cells
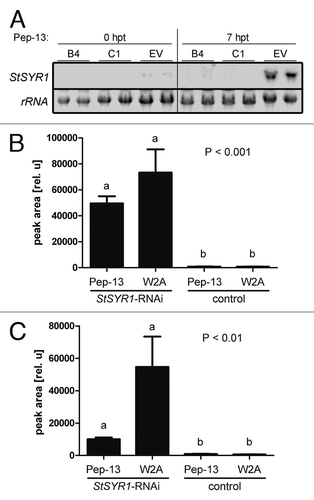
To determine possible effects of downregulation of StSYR1 expression on secretion processes, cell cultures of potato plants expressing the empty vector construct, as well as of two individual StSYR1-RNAi lines were initiated.Citation10 Northern blot analyses of cell cultures elicited with Pep-13 confirmed the downregulation of StSYR1 transcript accumulation in the RNAi lines (). For the untargeted metabolite profiling approach, cell cultures grown for four days were treated with Pep-13 or its inactive analog W2A as a control. After centrifugation, the media supernatants were directly used for untargeted profiling via LC-MS-measurements.Citation12 Interestingly, no compounds missing in StSYR1-RNAi samples compared with those of the control cultures could be detected. However, two metabolites, strongly accumulating in RNAi samples but not the control samples, were identified, independent of the treatment (). This finding implicates that StSYR1 might indeed be involved in the regulation of secretion processes, although the direct mode of action still needs to be uncovered. Nevertheless, one might speculate that the decrease of callose-containing papillae beneath penetration sites of P. infestans in StSYR1-RNAi plantsCitation1 and the delay of papillae formation in the Arabidopsis pen1 mutant at Bgh penetration sites,Citation13 points to the main role of these proteins related to the defense response, which is the delivery of secondary cell wall material to penetration sites. This is supported by their plasma membrane localization and the focal movement to penetration sites shown for AtPEN1.Citation14
Figure 4. Differential secretion of metabolites in StSYR1-RNAi cell cultures. Cell cultures were grown for four days in the dark with continuous shaking at 20°C. (A) Individual cultures were elicited with 10 nM Pep-13 and harvested by centrifugation at the time points indicated. Total RNA was isolated and used for northern blot hybridization with a radioactively labeled StSYR1-specific probe. Ethidium bromide-stained gels are depicted to show equal loading. EV, empty vector-transformed cell culture; B4, C1, independent StSYR1-RNAi cell cultures; hpt, hours post treatment. (B and C) Cell cultures were elicited with 10 nM Pep-13, media were harvested 24 h post elicitation by filtration and used for LC-MS measurements. Two differentially occurring peaks are plotted. Experiments were performed three times with similar results.
Acknowledgments
The authors would like to thank Sylvia Krüger for cell culture work, Birgit Ortel for help with the JA measurements and Thomas Franz for taking care of the greenhouse plants. This work was funded by the DFG (SFB 648, TP A4) and GABI Future (Papatomics).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Eschen-Lippold L, Landgraf R, Smolka U, Schulze S, Heilmann M, Heilmann I, et al. Activation of defense against Phytophthora infestans in potato by down-regulation of syntaxin gene expression. New Phytol 2012; 193:985 - 96; http://dx.doi.org/10.1111/j.1469-8137.2011.04024.x; PMID: 22243492
- Leyman B, Geelen D, Quintero FJ, Blatt MR. A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 1999; 283:537 - 40; http://dx.doi.org/10.1126/science.283.5401.537; PMID: 9915701
- Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature 2003; 425:973 - 7; http://dx.doi.org/10.1038/nature02076; PMID: 14586469
- Zhang Z, Feechan A, Pedersen C, Newman MA, Qiu JL, Olesen KL, et al. A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. Plant J 2007; 49:302 - 12; http://dx.doi.org/10.1111/j.1365-313X.2006.02961.x; PMID: 17241452
- Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 2011; 62:3321 - 38; http://dx.doi.org/10.1093/jxb/err031; PMID: 21357767
- Robert-Seilaniantz A, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 2011; 49:317 - 43; http://dx.doi.org/10.1146/annurev-phyto-073009-114447; PMID: 21663438
- Halim VA, Eschen-Lippold L, Altmann S, Birschwilks M, Scheel D, Rosahl S. Salicylic acid is important for basal defense of Solanum tuberosum against Phytophthora infestans. Mol Plant Microbe Interact 2007; 20:1346 - 52; http://dx.doi.org/10.1094/MPMI-20-11-1346; PMID: 17977146
- Halim VA, Altmann S, Ellinger D, Eschen-Lippold L, Miersch O, Scheel D, et al. PAMP-induced defense responses in potato require both salicylic acid and jasmonic acid. Plant J 2009; 57:230 - 42; http://dx.doi.org/10.1111/j.1365-313X.2008.03688.x; PMID: 18801014
- Brunner F, Rosahl S, Lee J, Rudd JJ, Geiler C, Kauppinen S, et al. Pep-13, a plant defense-inducing pathogen-associated pattern from Phytophthora transglutaminases. EMBO J 2002; 21:6681 - 8; http://dx.doi.org/10.1093/emboj/cdf667; PMID: 12485989
- Halim VA, Hunger A, Macioszek V, Landgraf P, Nürnberger T, Scheel D, et al. The oligopeptide elicitor Pep-13 induces salicylic acid-dependent and -independent defense reactions in potato. Physiol Mol Plant Pathol 2004; 64:311 - 8; http://dx.doi.org/10.1016/j.pmpp.2004.10.003
- Göbel C, Feussner I, Hamberg M, Rosahl S. Oxylipin profiling in pathogen-infected potato leaves. Biochim Biophys Acta 2002; 1584:55 - 64; PMID: 12213493
- Böttcher C, Westphal L, Schmotz C, Prade E, Scheel D, Glawischnig E. The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 2009; 21:1830 - 45; http://dx.doi.org/10.1105/tpc.109.066670; PMID: 19567706
- Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 2004; 15:5118 - 29; http://dx.doi.org/10.1091/mbc.E04-02-0140; PMID: 15342780
- Kwon C, Neu C, Pajonk S, Yun HS, Lipka U, Humphry M, et al. Co-option of a default secretory pathway for plant immune responses. Nature 2008; 451:835 - 40; http://dx.doi.org/10.1038/nature06545; PMID: 18273019