Abstract
Intracellular localization of stress induced reactive oxygen species (ROS) has emerged as an important aspect towards understanding of cellular responses to environmental stimuli. Our recent study published in the PNAS (103:18008–13)Citation1 shows that NaCl-induced ROS appear within endosomes on the way to tonoplast as part of the vacuolar vesicle trafficking. In addition to showing ROS damage to the tonoplast, this finding may shed light upon recently reported aspects of root water relations during salt stress, suggesting a new signaling role for intracellular ROS in Arabidopsis root cells, during salt stress: ROS that are compartmentalized in endosomes are delivered by the vacuolar vesicle trafficking pathway to the tonoplast, resulting in oxidative gating of TIPs water channels. The closure of the tonoplast aquaporins contributes to the observed reduction in root hydraulic conductivity during salt stress.
One of the major plant responses to abiotic stresses is generation of reactive oxygen species (ROS). The intracellular localization of ROS has become an important characteristic in the cellular responses to environmental stimuli.Citation2 The intracellular ROS signaling aspects were recently highlighted by Rhee, who defined the intracellular production of H2O2 as “a necessary evil for cell signaling”.Citation3 In Arabidopsis roots, H2O2 is produced by an NADPH oxidase (Nox) that becomes activated within minutes of salt stress.Citation4 Inhibition of Nox, either genetically or biochemically, resulted in increased salt sensitivity (unpublished data), indicating its significant role in salt stress signaling. Some important questions that remain are how is the signal delivered, and where is it targeted to?
Recently it was suggested that the vesicle trafficking machinery holds the potential for regulating plant responses to different environmental stresses.Citation5 This may be a result of membrane recyclingCitation6 or improved delivery of essential cargo to its destination, or of traffic-associated signal transduction.Citation7,Citation8
In the above paper Leshem et al. have studied the role of final step of the vacuolar trafficking on plant salt tolerance. The pathway terminates by vesicle docking and fusion to the tonoplast that is regulated by interaction of a v-SNARE (vesicle-soluble N-ethylm aleimide-sensitive factor attachment protein receptor) with target-SNARE. In Arabidopsis this process is regulated by a small gene family of v-SNAREs designated VAMP7C (vesicle associated membrane proteins) that consists of four genes, designated AtVAMP711–714 (Carter et al., 2004). Reduced expression of AtVAMP7C expression was achieved by transformation of plants with antisense AtVAMP711.
Analysis of ROS production by confocal microscopy showed that salt induced H2O2 was located in endosomes which are trafficked to the central vacuole. Transgenic plants that expressed the antisense AtVAMP7C showed a striking phenotype, in which H2O2 was caged within mega-vesicles that did not fuse to the tonoplast and remained in the cytoplasm (). Surprisingly these lines exhibited an improved salt resistance as compared to the wild type plants. The antisense lines also exhibited better vacuolar functioning, including maintenance of tonoplast pH and reduced vacuolar calcium release. An increased calcium-dependent protein kinase activity upon salt stress was observed in these lines too.
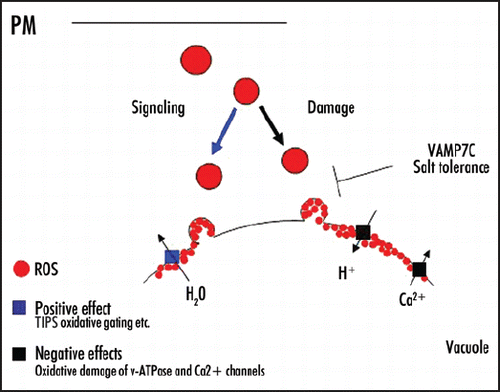
However, delivery of ROS to the tonoplast by an endogenous vacuolar trafficking pathway during salt stress suggests an adaptive role in stress tolerance. One possibility is the regulation of vacuolar water status, which holds most of mature cells' water content by oxidative gating of water channels. Salt stress was recently shown to decrease the hydraulic conductivity of roots, which is regulated by decreased expression of root aquaporins.Citation9 Since H2O2 was also shown to reduce root water transport,Citation10,Citation11 the localization of H2O2 on the tonoplast of root cells during salt stress (see ), may add another level of regulation by oxidative gating of the tonoplast intrinsic proteins (TIPs). This may represent a new intracellular communication pathway in which the signaling molecule is isolated from cell compartments in endosomes, due to its damaging nature. Although H2O2 was thought to be able to diffuse freely across membranes, recent studies showed that many membranes are poorly permeable to H2O2.Citation12 Thus, caging the ROS in vesicles may protect the cytosol while serving as a shuttle carrier to deliver the signaling molecule by vacuolar trafficking to its destination, where the ROS are released and act to close the vacuolar aquaporins (TIPs).
The signaling effect on TIPs occurs in parallel with an oxidative damage to the tonoplast. Thus, scavenging activity and shut-down of the VAMP7C pathway is needed to prevent excessive damage to tonoplast (see ). Interestingly, a microarray-based analysis of the AtVAMP7C family showed a strong down regulation of three out of four genes in wild type roots during salt stress, suggesting evolutionary molecular adaptation that down-regulates the vacuolar trafficking during salt stress.
Figures and Tables
Figure 1 Intracellular localization of NaCl induced ROS. Simultaneous double stain of ROS (H2DCFDA, green) and membrane tracer dye (red) during early minutes of salt stress in Arabidopsis root cells of wild type (wt) (A) and AtVAMP711 antisense transgenics (B). ROS are located within endosomes which are trafficked to the vacuolar membrane. Due to the reduced expression of the v-SNARE AtVAMP7C in the transgenic plants, ROS are precluded from the tonoplast and remain within cytoplasmic mega vesicles (B, inset, scale bar = 1 µm).
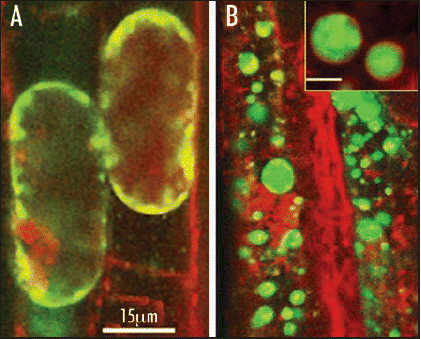
Figure 2 Model. Suggested mode of action for intracellular ROS localization during early events of salt stress in Arabidopsis root cells: In order to maintain vacuolar water status and reduce root hydraulic conductivity, ROS (red dots) are isolated from cell compartments in endosomes and delivered to the tonoplast via vacuolar vesicle trafficking, where oxidative gating of TIPs (blue square) occurs. After oxidative damage accumulates the vacuolar trafficking is shut down by the reduced expression of the AtVAMP7C genes.
References
- Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A. Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA 2006; 103:18008 - 18013
- Fedoroff N. Redox regulatory mechanisms in cellular stress responses. Ann Bot 2006; 98:289 - 300
- Rhee SG. Cell signaling: H2O2, a necessary evil for cell signaling. Science 2006; 312:1882 - 1883
- Mazel A, Leshem Y, Tiwari BS, Levine A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol 2004; 134:118 - 128
- Levine A. Regulation of stress responses by intracellular vesicle trafficking?. Plant Physiol Biochem 2002; 40:531 - 535
- Levine A, Belenghi B, Damari-Weisler H, Granot D. Vesicle associated membrane protein of Arabidopsis suppresses Bax-induced apoptosis in yeast downstream of oxidative burst. J Biol Chem 2001; 276:46284 - 46289
- Surpin M, Raikhel N. Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol 2004; 5:100 - 109
- Sutter JU, Campanoni P, Tyrrell M, Blatt MR. Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ Channel at the Plasma Membrane. Plant Cell 2006; 18:935 - 954
- Boursiac Y, Chen S, Luu DT, Sorieul M, van den Dries N, Maurel C. Early effects of salinity on water transport in arabidopsis roots: Molecular and cellular features of aquaporin expression. Plant Physiol 2005; 139:790 - 805
- Aroca R, Amodeo G, Fernandez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ. The role of Aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 2005; 137:341 - 353
- Ye Q, Steudle E. Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environm 2006; 29:459 - 470
- Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 2007; 282:1183 - 1192