Abstract
We have recently shown that an inhibition of sterol synthesis by fenpropimorph leads to an accumulation of sterol precursors, hydroxypalmitic acid-containing glucosylceramides and detergent resistant membranes in the Golgi bodies instead of the plasma membrane, suggesting that the individual molecules or the microdomains were blocked in the Golgi. These results and others from several eukaryotic models link lipid metabolism with membrane morphodynamics that are involved in membrane trafficking. Focus has been expanded to other lipid families, and numerous evidences are given showing lipids and lipid-modifying enzymes as key regulators of membrane homeostasis which can strongly regulate membrane morphodynamics and therefore trafficking. Beside protein-based machineries, lipid-based machineries are also shown as crucial regulatory forces involved in protein transport and sorting.
In the plant secretory pathway, it was shown that sterols, glucosylceramides, and the unsaturation degree of lipid acyl chains are critical for lipid microdomain formation at the cell surface.Citation1 Inhibition of sterol synthesis by fenpropimorph led to an accumulation of sterol precursors, glucosylceramides and detergent resistant membranes in the Golgi instead of the plasma membrane, suggesting that either individual molecules or microdomains were blocked in the Golgi. These results raised numerous questions and two additional aspects will be addressed here:
Are endomembrane morphodynamics linked to lipid metabolism?
Can we consider lipids as components of the cellular trafficking machineries?
Endomembrane Morphodynamics and Lipid Metabolism
More and more evidences highlight relationships between lipid metabolism and membrane morphodynamics. Blocking cyclopropylsterol maturation by fenpropimorph induced a fenestration of the Golgi.Citation2 Interestingly, disturbing Golgi morphology by brefeldin A reduced the synthesis of phytosterols.Citation3 Relationships may therefore exist between sterol metabolism and Golgi morphology in plant cells. In other eukaryotes, the sterol structure can affect membrane curvature and prepare a membrane for budding or fusion events.Citation4 For example, cholesterol is involved in the formation of post-Golgi secretory vesiclesCitation5 and may help to drive vesicle fusion by virtue of its intrinsic negative curvature.Citation6 However, cholesterol levels in the Golgi membranes must be tightly regulated since an excess of this molecule can vesiculate the Golgi complex itself.Citation7
Besides sterols, several other lipid families may affect membrane organisation. In animal cells, ceramides increase the disassembly of the Golgi induced by brefeldin A, indicating that ceramide levels could be critical for Golgi stability.Citation8 Pharmacological approaches using inhibitors of glucosylceramide synthase have suggested a role for sphingolipids in Golgi architecture.Citation9 High concentrations of sphingosine derived from ceramide hydrolysis can induce Golgi fragmentation.Citation10 Therefore, all these studies highlight a critical role for sphingolipids in Golgi morphodynamics as found for sterols.
Lysophospholipids, formed by phospholipases A2, can induce positive membrane curvatures and favor membrane tubulation.Citation11 Interestingly, inhibition of the reverse reaction by acyltransferases also leads to Golgi membrane tubulation.Citation12 Phospholipases A1-related enzymes can also induce dispersion of the Golgi complex and aggregation of ER membranes.Citation13 Inhibition of phospholipase D, which decreases phosphatidic acid (PA) but also phosphoinositide levels, can also affect the structural integrity of the Golgi complex in animal cells.Citation14 Besides the role of PA in stimulating phosphoinositide synthesis, PA and its derivative lyso-PA can regulate membrane curvature and therefore Golgi stability. In animal cells, an isoform of phospholipase D is concentrated to the rims of the Golgi stacks and could be involved in Golgi morphodynamics driving some aspects of vesicular transport from the Golgi.Citation15 In plant cells, inhibition of phospholipase D and therefore formation of PA in the pollen tube highly reduces the number of secretory vesicles, suggesting that PA levels but also phosphoinositides regulate pollen tube growth.Citation16,Citation17 In addition, we have observed that the inhibition of sterol synthesis by fenpropimorph was accompanied by an increase of PA in the endomembranes and especially the Golgi (), suggesting that elevated PA levels may drive the fenestration of Golgi stacks.Citation2 As a consequence, PA (and also diacylglycerol, see below) levels must be tightly controlled, and small variations may have dramatic effects.
Finally, it has recently been shown that the phospholipid-binding protein Nir2p (a Sec14-related protein) is involved in the control of diacylglycerol levels in mammalian Golgi membranes as in yeast,Citation18 and this could be related to the propensity of diacylglycerol to fission membranes. A Sec14p-nodulin PI-transfer protein from Arabidopsis has been shown to be a key sensor of polarized membrane growth in root hairs.Citation19
Therefore lipids and lipid-modifying enzymes must be considered as key molecular regulators of membrane domain formation and homeostasis which strongly regulate membrane morphodynamics and trafficking. A summary of the relationships between Golgi membrane morphodynamics and lipid metabolism is presented in .
Lipid Interactions with the Cellular Trafficking Machineries
As highlighted above, lipid-based machineries can regulate membrane structure through the control of membrane curvature, but can also be engaged in critical interactions with cargo proteins or proteins of the cellular trafficking machineries.
Protein sorting towards the secretory pathway may be linked to lipid-based sorting processes and specific interactions with acidic phospholipids.Citation20,Citation21 In this respect, it was interesting to determine a specific PS targeting to ER-derived domains in plant cells,Citation22 and that PS was required in the formation of ER-derived COPII vesicles in vitro.Citation23 In a similar way, short chain ceramides can interact with the binding of ARF to Golgi membranes and therefore affect the formation of COPI vesicles.Citation24 The yeast Oxysterol-binding protein (OSBP) Kes1p is a lipid receptor which may regulate Golgi secretory function through interactions with the ARF and Sec14 pathways.Citation25 In animal cells, OSBP-related proteins were found to interact with a syntaxin-like VAMP-associated protein-A, and to participate in the organisation of the COPII-dependent ER-Golgi pathway involved in protein and ceramide transport.Citation26 Finally, phospholipase D and phospholipase A1-related enzymes can interact with proteins of the COPII machinery to participate in the formation and organisation of ER export sites.Citation27,Citation28
It has recently been found that different SNAREs (proteins involved in membrane fusion) can have various affinities for specific lipid microdomains such as sterol- and sphingolipid-rich lipid rafts, and that the degree of their association with these domains could condition the efficiency of exocytosis.Citation29,Citation30 In addition, the basic domain of the synaptobrevin VAMP2, also involved in exocytosis, was found to strongly interact with PS.Citation31 Such interactions between lipids and proteins of the cellular transport machineries may contribute to both the specific targeting and function of these proteins.
It must be considered that at the time a lipid is synthesized, its physicochemical properties and structure will spontaneously allow or avoid specific interactions with other molecules (lipids or proteins) that will condition its association (and that of partners) within a microdomain or another, and that these features can be modified during transport through the secretory pathway. Beside protein-based machineries, lipid-based machineries are also crucial regulatory forces involved in protein trafficking and sorting through the secretory pathway.
Figures and Tables
Figure 1 Phosphatidic acid level in endomembranes of A. porrum seedlings treated with fenpropimorph. ER, endoplasmic reticulum; GA, Golgi membranes; PM, plasma membranes.
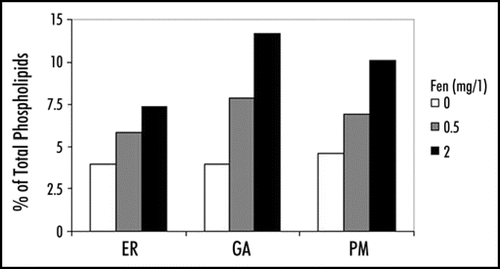
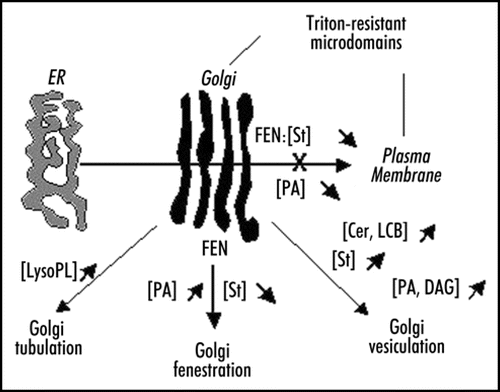
Figure 2 Lipid metabolism and Golgi morphodynamics. The different variations (up and down) in the concentrations of several lipids which influence the morphology of the Golgi are indicated. Most of the data come from studies carried out in animal models. The effect of fenpropimorph (FEN) on post-Golgi traffic and Golgi fenestration in plant cells is shown. Inhibition of PA formation is also affecting post-Golgi traffic in plant cells. Cer, ceramides; DAG, diacylglycerol; LCB, long chain bases of sphingolipids; LysoPL, lyso-phospholipids; PA, phosphatidic acid; St, sterols.
Addendum to:
References
- Laloi M, Perret AM, Chatre L, Melser S, Cantrel C, Vaultier MN, Zachowski A, Bathany K, Schmitter JM, Vallet M, Lessire R, Hartmann MA, Moreau P. Insights into the role of specific lipids in the formation and delivery of lipid microdomains to the plasma membrane of plant cells. Plant Physiol 2007; 143:1 - 12
- Hartmann MA, Perret AM, Carde JP, Cassagne C, Moreau P. Inhibition of the sterol pathway in leek seedlings impairs phosphatidylserine and glucosylceramide synthesis but triggers an accumulation of triacylglycerols. Biochim et Biophys Acta 2002; 1583:285 - 296
- Mérigout P, Képès F, Perret AM, Satiat-Jeunemaitre B, Moreau P. Effects of brefeldin A and nordihydroguaiaretic acid on endomembrane dynamics and lipid synthesis in plant cells. FEBS Lett 2002; 518:88 - 92
- Bacia K, Schwille P, Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc Natl Acad Sci 2005; 102:3272 - 3277
- Wang Y, Thiele C, Huttner WB. Cholesterol is required for the formation of regulated and constitutive secretory vesicles from the trans-Golgi network. Traffic 2000; 1:952 - 962
- Churchward MA, Rogasevskaia T, Höfgen J, Bau J, Coorssen JR. Cholesterol facilitates the native mechanism of Ca2+-triggered membrane fusion. J Cell Sci 2005; 118:4833 - 4848
- Grimmer S, Ying M, Wälchli S, van Deurs B, Sandvig K. Golgi vesiculation induced by cholesterol occurs by a dynamin- and cPLA2-dependent mechanism. Traffic 2005; 6:144 - 156
- Fukunaga T, Nagahama M, Hatsuzawa K, Tani K, Yamamoto A, Tagaya M. Implication of sphingolipid metabolism in the stability of the Golgi apparatus. J Cell Sci 2000; 113:3299 - 3307
- Nakamura M, Kuroiwa N, Kono Y, Takatsuki A. Glucosylceramide synthesis inhibitors block pharmacologically induced dispersal of the Golgi and anterograde membrane flow from the endoplasmic reticulum: Implication of sphingolipid metabolism in maintenance of the Golgi architecture and anterograde membrane flow. Biosci Biotech Biochem 2001; 65:1369 - 1378
- Hu W, Xu R, Zhang G, Jin J, Szulc ZM, Bielawski J, Hannun YA, Obeid LM, Mao C. Golgi fragmentation is associated with ceramide-induced cellular effects. Mol Biol Cell 2005; 16:1555 - 1567
- De Figueiredo P, Drecktrah D, Katzenellenbogen JA, Strang M, Brown WJ. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc Natl Acad Sci 1998; 95:8642 - 8647
- Drecktrah D, Chambers K, Racoosin EL, Cluett EB, Gucwa A, Jackson B, Brown WJ. Inhibition of a Golgi complex lysophospholipid acyltransferase induces membrane tubule formation and retrograde trafficking. Mol Biol Cell 2003; 14:3459 - 3469
- Nakajima KI, Sonoda H, Mizoguchi T, Aoki J, Arai H, Nagahama M, Tagaya M, Tani K. A novel phospholipase A1 with sequence homology to a mammalian Sec23p-interacting protein, p125. J Biol Chem 2002; 277:11329 - 11335
- Siddhanta A, Backer JM, Shields D. Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J Biol Chem 2000; 275:12023 - 12031
- Freyberg Z, Bourgoin S, Shields D. Phospholipase D2 is localized to the rims of the Golgi apparatus in mammalian cells. Mol Biol Cell 2002; 13:3930 - 3942
- Potocky M, Elias M, Profotova B, Novotna Z, Valentova O, Zarsky V. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta 2003; 217:122 - 130
- Monteiro D, Liu Q, Lisboa S, Scherer GEF, Quader H, Malho R. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J Exp Bot 2005; 416:1665 - 1674
- Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol 2005; 7:225 - 234
- Vincent P, Chua M, Nogue F, Fairbrother A, Mekeel H, Xu Y, Allen N, Bibikova TN, Gilroy S, Bankaitis VA. A sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol 2005; 168:801 - 812
- Ceppi P, Colombo S, Francolini M, Raimondo F, Borgese N, Masserini M. Two tail-anchored protein variants, differing in transmembrane domain length and intracellular sorting, interact differently with lipids. Proc Natl Acad Sci 2005; 102:16269 - 16274
- Lev S. Lipid homoeostasis and Golgi secretory function. Biochem Soc Trans 2006; 34:363 - 366
- Vincent P, Maneta-Peyret L, Cassagne C, Moreau P. Phosphatidylserine delivery to endoplasmic reticulum-derived vesicles of plant cells depends on two biosynthetic pathways. FEBS Lett 2001; 498:32 - 36
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 1998; 93:263 - 275
- Abousalham A, Hobman TC, Dewald J, Garbutt M, Brindley DN. Cell-permeable ceramides preferentially inhibit coated vesicle formation and exocytosis in Chinese hamster ovary compared with Madin-darby canine kidney cells by preventing the membrane association of ADP-ribosylation factor. Biochem J 2002; 361:653 - 661
- Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol 2002; 157:63 - 78
- Wyles JP, McMaster CR, Ridgway ND. VAMP-associated protein-A (VAP-A) interacts with the oxysterol binding protein (OSBP) to modify export from the endoplasmic reticulum. J Biol Chem 2002; 277:29908 - 29918
- Pathre P, Shome K, Blumental-Perry A, Bielli A, Haney CJ, Alber S, Watkins SC, Romero G, Aridor M. Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export. EMBO J 2003; 22:4059 - 4069
- Shimoi W, Ezawa I, Nakamoto K, Uesaki S, Gabreski G, Aridor M, Yamamoto A, Nagahama M, Agaya M, Tani K. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem 2005; 280:10141 - 10148
- Salaün C, Gould GW, Chamberlain LH. The SNARE proteins SNAP-25 and SNAP-23 display different affinities for lipid rafts in PC12 cells. J Biol Chem 2005; 280:1236 - 1240
- Salaün C, Gould GW, Chamberlain LH. Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J Biol Chem 2005; 280:19449 - 19453
- De Haro L, Quetglas S, Iborra C, Leveque C, Seagar M. Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion. Biol Cell 2003; 95:459 - 464