Abstract
The interaction of plants with bacterial pathogens involves the manipulation of programmed cell death (PCD) pathways. During a resistance interaction PCD is induced in a process termed the hypersensitive response (HR) which may function to limit pathogen spread. In a susceptible plant-pathogen interaction the pathogen both inhibits and induces host PCD depending on the infection stage. Genes/pathways regulating PCD in plants have been difficult to identify due to a lack of homologous sequences in plants for mammalian genes that control apoptosis and possibly due to functional redundancy. Our labs study plant PCD pathways and bacterial speck disease in tomato which is caused by Pseudomonas syringae pv. tomato (Pst). We recently identified the tomato protein kinases Pdk1 and Adi3 as negative regulators of plant PCD. The plant Pdk1/Adi3 pathway appears to function similarly to the Pdk1/PKB (Akt) pathway in mammals which functions as a major apoptosis negative regulation pathway. Here we discuss regulation of Pdk1/Adi3 and targeting of this pathway during the tomato-Pst interaction for modulation of host PCD.
Plant pathogenic bacteria, such as Pseudomonas syringae pv. tomato (Pst), promote disease in hosts by injecting effector proteins into the plant cell using a type III secretion system (TTSS). Once inside the plant cell, these effector proteins suppress immunity pathways and promote certain host susceptibility factors.Citation1,Citation2 While the direct targets of most effector proteins are unknown, recently some have been shown to act as suppressors of immunity-associated programmed cell death (PCD).Citation3–Citation5 Plants have evolved resistance (R) proteins to recognize some pathogen effector proteins. This recognition leads to massive changes in gene expression and resistance to the pathogen.Citation6,Citation7 The hypersensitive response (HR) is a part of resistance and includes rapid cell death and associated cell wall thickening at the site of attempted infection that appears to limit pathogen spread and access to nutrients. The host cell death which occurs during the HR is a form of PCD and occurs within 12 hours of pathogen recognition.Citation8 From this knowledge it is obvious that manipulation of host PCD pathways plays a very important role during both immunity and disease. However, it has been difficult to identify plant genes involved in regulating PCD let alone determining if they are specifically manipulated during plan-pathogen interactions.
Bacterial speck disease of tomato is caused by Pst and the Pst effector protein AvrPto is known to promote disease.Citation6 However, tomato lines containing the Ser/Thr protein kinase R protein Pto are resistant to Pst through recognition of AvrPto by Pto.Citation6 We are studying genes involved in regulating PCD pathways in tomato and how they are affected during the Pst-tomato interaction.
Plant PCD Genes
The search for plant genes involved in PCD has mostly revealed genes that have an indirect role in regulating PCDCitation9 and not direct pathway regulators. Searching for direct plant PCD regulating genes based on sequence identity to mammalian apoptosis regulators (e.g.: PKB/Akt and Bcl-2 family members) indicates such sequences are not obviously present in plants. However, advanced bioinformatic searches have revealed the presence of some of these types of sequences and functions in plants.Citation10 It remains to be determined if these methods will identify more mammalian-like PCD regulators.
We recently used a combination of methods to identify two protein kinases, Adi3 and Pdk1, from tomato that act as negative regulators of plant PCD.Citation11 Adi3, a Ser/Thr protein kinase, was isolated based on its interaction with the tomato R protein Pto and the Pst effector protein AvrPto.Citation12 Adi3 belongs to the AGC family of protein kinases. A central regulator of AGC kinases, the Ser/Thr protein kinase 3-phosphoinositide-dependent protein kinase-1 (Pdk1), was shown to activate Adi3 by phosphorylation at Ser539.Citation11 Using both virus induced gene silencing (VIGS) and an inhibitor of Pdk1, Adi3 and Pdk1 were shown to be negative regulators of PCD.Citation11 Additionally, Adi3 was able to suppress HR cell death induced by a constitutive form of Pto.Citation11
Adi3 was also connected to pathogen-associated host cell death pathways. Using VIGS, Adi3 activity was shown to signal through MAPKKKa,Citation11 a MAPKKK known to regulate PCD during both resistant and susceptible plant-pathogen interactions.Citation13 Additionally, Pto was shown to phosphorylate Adi3 independent of AvrPto.Citation11 The interaction of Adi3 with Pto/AvrPto and the connection of its function with pathogen-associated cell death suggests the Pdk1/Adi3 cell death regulation pathway is manipulated during pathogen interactions to bring about cell death.
Regulation of the Pdk1/Adi3 Pathway
In mammalian systems, Pdk1 regulates substrates in both a stimulated and constitutive manner.Citation14 For example, stimulated production of phosphatidylinositol 3,4,5-triphosphate (PtdIns(3,4,5)P3) localizes Pdk1 to the plasma membrane where it can activate substrates that also bind PtdIns(3,4,5)P3 such as PKB.Citation15,Citation16 The pleckstrin homology (PH) domain of Pdk1 and PKB is responsible for PtdIns(3,4,5)P3 binding.Citation16,Citation17 Recently it has been shown that for plant Pdk1 phosphatidic acid (PA), not PtdIns(3,4,5)P3, binds the PH domain and acts as the lipid activator.Citation18 Pdk1 also activates cytoplasmically localized substrates and is thought to be anchored in the cytoplasm by PH domain binding to inositol phosphates.Citation17 Thus, PA activation of Adi3 through Pdk1 phosphorylation seems unlikely since Adi3 is predicted to be soluble and does not contain a PH domain. It is yet to be determined if plant Pdk1 can bind inositol phosphates for cytoplasmic localization. Cytoplasmically localized Pdk1 may constitutively activate Adi3 and control of Adi3 activity would most likely be at the level of phosphatases, as has been shown for other proteins that are constitutively phosphorylated by Pdk1.Citation14
A Model for directed loss of Adi3 function in Pathogen-associated cell death
Our data support a model () that when cells are challenged by Pst the interaction of Adi3 with Pto/AvrPto interferes with the negative regulatory function of Adi3 leading to cell death. Pto phosphorylates Adi3Citation11 and it is possible this event inhibits Adi3 function. Our in vitro data indicate this phosphorylation does not require AvrPto indicating that the role of AvrPto, which is known to be localized to the plasma membraneCitation6 might be to recruit Pto/Adi3 to that cellular location. Thus, a change in the localization of Adi3 to the plasma membrane would not allow for cytoplasmically localized Pdk1 to activate Adi3. This, in combination with Pto phosphorylation of Adi3 may lead to a loss of Adi3 function and bring about cell death.
Our data leave unresolved the question of whether Adi3 inactivation during pathogen interactions underlies cell death associated with immunity or with disease. The association of MAPKKKα with Adi3 suggests that Adi3 could be a target during both resistance and disease. However, recent evidence indicates that kinase activity of Pto is not required for HR-associated cell death.Citation19 This suggests that if Pto phosphorylation of Adi3 is biologically significant it may play a role in disease-associated cell death.
The discovery of the Pdk1/Adi3 pathway provides new perspectives on PCD regulation and manipulation during plant-pathogen interactions and indicates that mammalian-like PCD regulators are present in plants. Our studies indicate that Adi3 is the functional, not sequence, homolog of mammalian PKB, a Pdk1 activated negative regulator of apoptosis,Citation20 even though the two proteins share only 21.4% identity.Citation11 This may indicate that other mammalian-like PCD regulators exist in plants but their discovery will require functional studies and not sequence-based analysis.
Figures and Tables
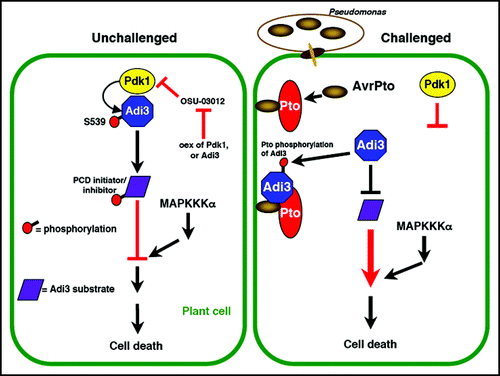
Figure 1 Model for manipulation of Pdk1/Adi3 cell death regulation during pathogen interaction. In unchallenged plant cells, left, Adi3 is activated by Pdk1 phosphorylation at S539. Adi3 then acts to negatively regulate cell death by activating PCD inhibitors or inactivating PCD initiators. MAPKKKα acts downstream in a parallel connected pathway.Citation11 The Pdk1 inhibitor OSU-03012 induces cell death that can be attenuated by Pdk1 or Adi3 overexpression.Citation11 In Pst challenged cells, right, Adi3 interaction with Pto/AvrPto changes Adi3 cellular localization eliminating Pdk1 activation of Adi3. Thus, Adi3 can not act on downstream substrates leading to loss of cell death control and bringing about cell death. Pto phosphorylation of Adi3 may contribute to this inactivation of Adi3.
Addendum to:
References
- Abramovitch RB, Martin GB. Strategies used by bacterial pathogens to suppress plant defenses. Curr Opin Plant Biol 2004; 7:356 - 364
- Cohn JR, Martin GB. Pseudomonas syringae pv. tomato type III effectors AvrPto and AvrPtoB promote ethylene-dependent cell death in tomato. Plant J 2005; 44:139 - 154.
- Abramovitch RB, Anderson JC, Martin GB. Bacterial elicitation and evasion of plant innate immunity. Nat Rev Mol Cell Biol 2006; 7:601 - 611
- Abramovitch RB, Janjusevic R, Stebbins CE, Martin GB. Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proc Natl Acad Sci USA 2006; 103:2851 - 2856
- Janjusevic R, Abramovitch RB, Martin GB, Stebbins CE. A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science 2006; 311:222 - 226
- Pedley KF, Martin GB. Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu Rev Phytopathol 2003; 41:215 - 243
- Mysore KS, Crasta OR, Tuori RP, Folkerts O, Swirsky PB, Martin GB. Comprehensive transcript profiling of Pto- and Prf-mediated host defense responses to infection by Pseudomonas syringae pv. tomato. Plant J 2002; 32:299 - 315
- Goodman RN, Novacky AJ. The hypersensitive reaction in plants to pathogens: A resistance phenomenon 1994; St. Paul The American Phytopathological Society Press
- Lam E. Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 2004; 5:305 - 315
- Doukhanina EV, Chen S, van der Zalm E, Godzik A, Reed J, Dickman MB. Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J Biol Chem 2006; 281:18793 - 18801
- Devarenne TP, Ekengren SK, Pedley KF, Martin GB. Adi3 is a Pdk1-interacting AGC kinase that negatively regulates plant cell death. EMBO J 2006; 25:255 - 265
- Bogdanove AJ, Martin GB. AvrPto-dependent Pto-interacting proteins and AvrPto-interacting proteins in tomato. Proc Natl Acad Sci USA 2000; 97:8836 - 8840
- del Pozo O, Pedley KF, Martin GB. MAPKKKα is a positive regulator of cell death associated with both plant immunity and disease. EMBO J 2004; 23:3072 - 3082
- Belham C, Wu S, Avruch J. Intracellular signalling: PDK1-a kinase at the hub of things. Curr Biol 1999; 9:R93 - R96
- Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 2004; 15:161 - 170
- Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, Cohen P, Alessi DR, Lucocq J. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J 1999; 337:575 - 583
- Komander D, Fairservice A, Deak M, Kular GS, Prescott AR, Peter Downes C, Safrany ST, Alessi DR, van Aalten DM. Structural insights into the regulation of PDK1 by phosphoinositides and inositol phosphates. EMBO J 2004; 23:3918 - 3928
- Anthony RG, Henriques R, Helfer A, Meszaros T, Rios G, Testerink C, Munnik T, Deak M, Koncz C, Bögre L. A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J 2004; 23:572 - 581
- Wu AJ, Andriotis VM, Durrant MC, Rathjen JP. A patch of surface-exposed residues mediates negative regulation of immune signaling by tomato Pto kinase. Plant Cell 2004; 16:2809 - 2821
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2:489 - 501