Abstract
Plants respond to local herbivory or pathogen infection with phenotypic changes, which reduce the danger of future attack. This so-called induced resistance is usually not restricted to the attacked plant organ but is also expressed in distant, so far undamaged parts of the plant. Signaling compounds such as jasmonic acid and salicylic acid have been discovered that move within the plant via the xylem or the phloem and elicit the resistance, thus acting as plant hormones. We now found that volatiles released in response to herbivore damage are required to elicit extrafloral nectar secretion in other parts of the same plant. Extrafloral nectar attracts ants and other carnivorous arthropods and serves as an effective indirect defense against herbivores. So called green leaf volatiles are released within minutes in response to tissue damage and were among the compounds that induced nectar secretion in yet undamaged parts of the damaged plant, but also in neighboring plants. Being gaseous and transported via the air, green leaf volatiles can serve a rapid within-plant communication, which moves much faster from one plant organ to the other than any plant-internal compound.
Induced plant resistance traits are expressed in response to an eliciting attack. While pathogenesis-related proteins and phytoalexins are important means of induced systemic resistance to pathogens,Citation1,Citation2 induced resistance to herbivores comprises direct defenses and traits that improve the plant's interaction with the third trophic level.Citation3 For instance, extrafloral nectar (EFN) is usually secreted on vegetative organs and attracts ants and other carnivorous arthropods, thereby increasing the predation pressure on herbivores.Citation4 EFN secretion increases in response to mechanical damage or herbivore feedingCitation5–Citation9 and thus represents an induced indirect defense. Similarly, herbivore-induced volatiles (HI-VOCs) are released in response to herbivore damage and serve as defensive means by attracting carnivorous arthropods such as parasitoidic wasps.Citation10–Citation12 While the synthesis of PR proteins and phytoalexins depends on salicylic acid moving within the phloem and eliciting the systemic resistance,Citation13 jasmonic acid (JA) has been identified as a hormone that is both sufficient and required to elicit induced defenses to herbivores.Citation14–Citation16
Systemic plant responses, i.e., the induction of resistance in yet undamaged plant parts, therefore are generally believed to be mediated by signaling molecules that move within the plant body. Recently, however, we and another group demonstrated that HI-VOCs can be both required and sufficient to elicit defensive responses. By mechanically clipping sagebrush leaves and bagging damaged twigs, Karban and coworkersCitation17 found that air flow from damaged to undamaged parts was required to elicit resistance in yet undamaged parts of the same plant. While Karban et al.Citation17 focused on net plant damage, we made use of the existence of both EFN and HI-VOCs in the same plant species, Lima bean (Phaseolus lunatus), in order to directly study a single defensive response. EFN secretion by Lima bean responds to mechanical damage and herbivore feeding and can be induced also by exogenous application of JA.Citation18 HI-VOCs can both induceCitation19 and primeCitation20 EFN secretion by undamaged Lima bean plants, thus causing a plant-plant communication phenomenon.
Since Baldwin and Schultz in 1983 for the first time reported ‘plant-plant communication’,Citation21 it has been controversially debated whether or not this phenomenon plays a rolein nature.Citation22 One question usually raised was how a plant trait can be evolutionarily stable that serves the neighboring at the cost of the volatile-emitting plant. The usual explanation was plants emit HI-VOCs as a means of indirect defense, and their neighbors only ‘eavesdrop’ on this information on the status of attack of a plant. Interestingly, plants can also be ‘primed’ by HI-VOCs, i.e., their response to herbivore attack can be much more pronounced when they before have been exposed to HI-VOCs.Citation20,Citation23–Citation25
The observations by Karban et al.Citation17 and our most recent studyCitation26 now demonstrate a new function of HI-VOCs. We exposed Lima bean plants growing naturally in the field in Mexico to beetle-damaged tendrils and observed a reduced degree of herbivory and increased growth rates of exposed as compared to control tendrils. EFN secretion can be induced by HI-VOCs released from damaged Lima bean tendrilsCitation19 and was likely the major reason of this effect. In consecutive studies, we damaged only some leaves (hereinafter called ‘emitters’) and quantified EFN secretion by other, yet undamaged leaves (‘receivers’) of the same shoot and of other, neighboring shoots. By bagging the emitter leaves with plastic foil or leaving them unbagged we could distinguish among responses of receiver leaves that were either exposed or not exposed to HI-VOCs released from the damaged leaves. Undamaged leaves increased their EFN secretion significantly when exposed to HI-VOCs of the neighboring leaves. This observation could be repeated in a second, independent experiment using beetle-damaged leaves and moving the air from those leaves towards—or away from—undamaged receivers by means of tubes and ventilators. Since damaged emitter and undamaged receiver leaves inserted on the same shoot, any plant- internal signal produced in the damaged leaves and then transported via the plant sap should have affected the undamaged receivers in all cases. That the receivers responded only when being exposed to the volatiles released from the emitters demonstrates that HI-VOCs released by damaged leaves can be a signal inducing a defensive response in yet undamaged leaves of the same plant.
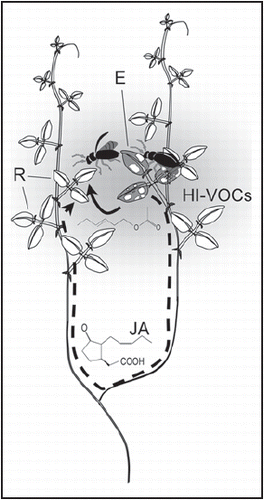
HI-VOCs exhibit a hormone-like function in within-plant signaling, and plant-plant communication appears to result from plants being ‘eavesdropping’ on what is within-plant signaling worn on the outside. In spite of this potential ‘information parasitism’, the benefits of within-plant signaling mediated by external, volatile compounds are manifold. Individual HI-VOCs identified so far to cause priming or induction of defense in undamaged plants include (3Z)-hex-3-enyl acetateCitation19 and several structurally related C6-volatiles,Citation23,Citation27–Citation30 i.e., the substances involved in this phenomenon are typical green leaf volatiles and thus are released within minutes after tissue disruption. HI-VOCs-mediated within-plant signaling might thus be much faster in eliciting a systemic response than any signal that is transported as classical hormone in phloem or xylem. Moreover, herbivores often are highly mobile and their movements among plant parts do not necessarily follow the anatomy of the plant. Particularly in a liana such as Lima bean, a plant-internal signal would be less efficient as long as it is transported through the shoots, since leaves located most closely in space might insert on different shoots and therewith at an anatomical distance of several meters. VOCs can serve as a cue to elicit EFN secretion in exactly those parts of another—or even the same—plant individual, where resistance actually is required: in the spatially (yet not necessarily anatomically) neighboring parts ().
Studies into systemic responses of plants to pathogens have so far not controlled air flow among plant parts and thus could not find the phenomenon as described by Karban et al.Citation17 and in our study.Citation26 Future studies must investigate to which degree herbivore or pathogen-induced volatile compounds are involved in the systemic responses of plants to local attacks. From the hypothesis given above we conclude that volatiles should be more important as resistance-eliciting means in cases of highly mobile attackers and less important in cases of attackers that only move on the plant surface or within the plant body.
Figures and Tables
Figure 1 Within-plant signaling by volatiles. Volatiles (shadowed area) fill the aerial space around an herbivore-damaged emitter leaf(E, marked grey) and can rapidly induce spatially neighboring receiver leaves (R), which might anatomically be very distant. Volatile-mediated signaling (bold arrow) that functions via green leaf volatiles such as the displayed (Z)-3-hexenyl acetate thus allows much shorter signaling ways from emitter to receiver leaf that molecules such as jasmonic acid (JA) which move within the plant veins (scattered arrow).
References
- Sticher L, Mauch-Mani B, Métraux JP. Systemic acquired resistance. Annu Rev Phytopathol 1997; 35:235 - 270
- van Loon LC. Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 1997; 103:753 - 765
- Karban R, Baldwin IT. Induced responses to herbivory 1997; Chicago and London University of Chicago Press
- Koptur S. Bernays EA. Extrafloral nectary-mediated interactions between insects and plants. Insect-plant interactions. IV 1992; Boca Raton CRC Press 81 - 129
- Heil M, Greiner S, Meimberg H, Krüger R, Noyer JL, Heubl G, Linsenmair KE, Boland W. Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature 2004; 430:205 - 208
- Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA 2001; 98:1083 - 1088
- Ness JH. Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia 2003; 134:210 - 218
- Stephenson AG. The role of the extrafloral nectaries of Catalpa speciosa in limiting herbivory and increasing fruit production. Ecology 1982; 63:663 - 669
- Wäckers FL, Zuber D, Wunderlin R, Keller F. The effect of herbivory on temporal and spatial dynamics of foliar nectar production in cotton and castor. Ann Bot 2001; 87:365 - 370
- De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature 1998; 393:570 - 573
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001; 291:2141 - 2144
- Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 1995; 92:4169 - 4174
- Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance. Science 1990; 250:1004 - 1006
- Boland W, Hopke J, Donath J, Nüske J, Bublitz F. Jasmonic acid and coronatin induce odor production in plants. Angew Chemie Intl Ed 1995; 34:1600 - 1602
- Farmer EE, Alméras E, Krishnamurthy V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 2003; 6:372 - 378
- Major IT, Constabel CP. Insect regurgitant and wounding elicit similar defense responses in Poplar leaves. Plant Signaling and Behavior 2007; 2:e1 - e3
- Karban R, Shiojiri K, Huntzinger M, McCall AC. Damage-induced resistance in sagebrush: Volatiles are key to intra- and interplant communication. Ecology 2006; 87:922 - 930
- Heil M. Induction of two indirect defenses benefits Lima bean (Phaseolus lunatus, Fabaceae) in nature. J Ecol 2004; 92:527 - 536
- Kost C, Heil M. Herbivore-induced plant volatiles induce an indirect defense in neighboring plants. J Ecol 2006; 94:619 - 628
- Heil M, Kost C. Priming of indirect defenses. Ecol Lett 2006; 9:813 - 817
- Baldwin IT, Schultz JC. Rapid changes in tree leaf chemistry induced by damage: Evidence for communication between plants. Science 1983; 221:277 - 279
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006; 311:812 - 815
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 2004; 101:1781 - 1785
- Kessler A, Halitschke R, Diezel C, Baldwin IT. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006; 148:280 - 292
- Ton J, D'Allesandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 2007; 49:16 - 26
- Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 2007; (publ. online 7. Mar 2007: doi:10.1073/pnas.0610266104)
- Bate NJ, Rothstein SJ. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 1998; 16:561 - 569
- Farag MA, Fokar M, Zhang HA, Allen RD, Paré PW. (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta 2005; 220:900 - 909
- Ruther J, Kleier S. Plant-plant signaling: Ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-Hexen-1-ol. J Chem Ecol 2005; 31:2217 - 2222
- Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol 2005; 46:1093 - 1102