Abstract
Redox regulation based on dithiol-disulphide interchange is an essential component of the control of chloroplast metabolism. In contrast to heterotrophic organisms, and non-photosynthetic plant tissues, chloroplast redox regulation relies on ferredoxin (Fd) reduced by the photosynthetic electron transport chain, thus being highly dependent on light. The finding of the NADPH-dependent thioredoxin reductase C (NTRC), a chloroplast-localized NTR with a joint thioredoxin domain, showed that NADPH is also used as source of reducing power for chloroplast redox homeostasis. Recently we have found that NTRC is also in plastids of non-photosynthetic tissues. Because these non-green plastids lack photochemical reactions, their redox homeostasis depends exclusively on NADPH produced from sugars and, thus, NTRC may play an essential role maintaining the redox homeostasis in these plastids. The fact that redox regulation occurs in any type of plastids raises the possibility that the functions of chloroplasts and non-green plastids, such as amyloplasts, are integrated to harmonize the growth of the different organs of the plant. To address this question, we generated Arabidopsis plants the redox homeostasis of which is recovered exclusively in chloroplasts, by leaf-specific expression of NTRC in the ntrc mutant, or exclusively in amyloplasts, by root-specific expression of NTRC. The analysis of these plants suggests that chloroplasts exert a pivotal role on plant growth, as expected because chloroplasts constitute the major source of nutrients and energy, derived from photosynthesis, for growth of heterotrophic tissues. However, NTRC deficiency causes impairment of auxin synthesis and lateral root formation. Interestingly, recovery of redox homeostasis of chloroplasts, but not of amyloplasts, was sufficient to restore wild type levels of lateral roots, showing the important signaling function of chloroplasts for the development of heterotrophic organs.
Post-translational modification, based on dithiol-disulphide interchange, constitutes a rapid and reversible mechanism of regulation, thus allowing the efficient adaptation of metabolism to environmental changes. Thioredoxins (Trxs), small proteins with a pair of conserved cysteine residues at their active site, play an essential role in protein disulphide reduction using reducing power provided by NADPH in a reaction catalyzed by an NADPH-dependent thioredoxin reductase (NTR).Citation1 The two-component NTR/Trx redox system is found in all types of organisms from bacteria to plants and animals. However, plants show remarkable peculiarities concerning redox regulation such as an unusually high number of genes encoding Trxs and Trx-like proteins.Citation2 Moreover, chloroplasts are equipped with a complex set of specific Trxs, which in addition use a chloroplast-specific ferredoxin-dependent thioredoxin reductase (FTR), which is different from the NTR present in heterotrophic tissues. Thus, in contrast to heterotrophic organisms, chloroplast redox regulation is highly dependent on light since it relies on ferredoxin reduced by the photosynthetic electron transport chain, rather than NADPH.
Recently, our group described a peculiar NTR with a joint Trx domain at the C-terminus, termed NTRC, which is exclusive for oxygenic photosynthetic organisms and is localized to chloroplasts.Citation3 NTRC is able to efficiently reduce disulphides of target proteins, such as 2-Cys peroxiredoxins using NADPH as source of reducing power. Thus NTRC behaves as an NTR/Trx system in a single polypeptide.Citation4-Citation6 Based on these results, a new scenario emerged according to which both reduced Fd and NADPH can be used to maintain redox homeostasis in the chloroplastCitation7 (). During the day both systems may have complementary functions. However, during the night, when reduced Fd becomes limiting, NADPH produced from sugars by the oxidative pentose phosphate pathway may be the major source of reducing power available and, thus, NTRC becomes essential to maintain chloroplast redox homeostasis. The fact that an Arabidopsis NTRC knock out mutant is hypersensitive to treatments of prolonged darkness and shows a more severe phenotype when grown under short-day photoperiodCitation4,Citation8 was taken as evidence in support of this proposal.
Figure 1. Two pathways operate to maintain redox homeostasis in chloroplasts of photosynthetic tissues, the FTR/Trx pathway, which relies directly on light, and the NTRC pathway, which uses NADPH produced either from sugars by the oxidative pentose phosphate pathway (OPPP), or from Fd/FNR. Both pathways may be complementary because FNR activity allows interchange of reduced Fd and NADPH. In plastids of non-photosynthetic tissues the FTR/Trx and NTRC pathways are also present but the only source of reducing power is NADPH produced by the OPPP. The metablolism of both types of plastids is interconnected by nutrients, such as sucrose, produced by photosynthesis in green tissues. Recent results suggest that chloroplast redox homeostasis might be important for auxin synthesis, serving as signal to harmonize the growth of photosynthetic and non-photosynthetic tissues in Arabidopsis seedlings.
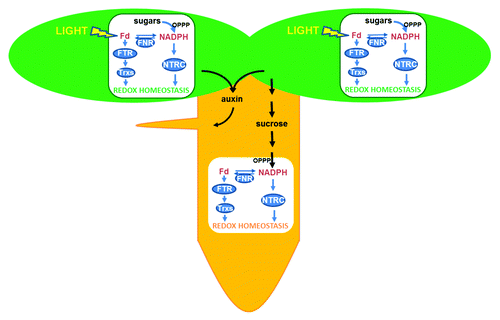
Besides the presence of chloroplasts in green tissues, plants also contain plastids in heterotrophic tissues. Indeed, a proteomics analysis performed in one such type of plastids, the amyloplasts from wheat grain endosperm, showed that these non-photosynthetic plastids are equipped with the components of FTR/Trx system,Citation9 suggesting the existence of redox regulation in non-photosynthetic plastids. This possibility raised the question of the source of reducing power to maintain redox homeostasis in these plastids, which have no photochemical reactions. The finding that redox regulation of ADP-glucose pyrophosphorylase, a key regulatory enzyme of starch synthesis, is impaired both in leaves and roots of the Arabidopsis NTRC knock out mutantCitation10 provided the first indication that NTRC might play an important function in maintaining redox homeostasis of non-photosynthetic plastids. Further analyses of the expression and localization of NTRC showed its expression in photosynthetic and non-photosynthetic tissues, the enzyme being localized in any type of plastid. Moreover, it could be shown that NTRC is involved in the control of the redox status of the 2-Cys Prxs not only in chloroplasts, but also in plastids of non-photosynthetic tissues,Citation11 thus lending support to the proposal that redox regulation is operative in any type of plastid. Therefore, all these new findings allow proposing a model according to which redox regulation is an important component of the function of plastids from green and heterotrophic tissues. While in chloroplasts this regulation relies on light (by the FTR/Trx pathway) or sugars (by the NTRC pathway), in non-photosynthetic plastids, which have no photochemical reactions, it relies exclusively on NADPH produced from sucrose metabolism by the oxidative pentose phosphate pathway, as depicted in . NTRC is able to use the reducing power of NADPH to reduce the cysteine residues at the active site of its Trx domain, allowing the direct conversion of NADPH into redox signals. Therefore, NTRC is an ideal enzyme to perform redox regulation. However, genes encoding FNR, FTR and Trxs, are also expressed in Arabidopsis roots,Citation11 though at low level, and this might allow a complementary pathway for redox regulation in plastids of non-photosynthetic tissues (). It is not yet known which of the two pathways is more relevant to maintain the redox homeostasis of these plastids.
The fact that any type of plastid, photosynthetic and non-photosynthetic, possesses the components for redox regulation raises the question whether plants have any kind of mechanism to coordinate their function and effect on plant growth. To address this question we have focused on a characteristic phenotype of the Arabidopsis ntrc mutant, which is the lower content of lateral roots.Citation11 Formation of lateral roots at early stages of seedling growth in Arabidopsis is largely under the control of auxins.Citation12 Interestingly, it has been shown that the ntrc mutant has a lower content of auxins,Citation8 showing that chloroplast redox homeostasis affects auxin synthesis, which might be the reason for the lateral root formation phenotype of this mutant. We have addressed whether the deficiency of lateral roots in the ntrc mutant is caused by impaired redox homeostasis of leaf chloroplasts or root amyloplasts. Arabidopsis transgenic plants expressing NTRC exclusively in leaves, thus recovering redox homeostasis of chloroplasts but not of root amyloplasts, recovered wild type levels of lateral roots. In contrast, plants expressing NTRC exclusively in roots, thus recovering redox homeostasis of amyloplasts, but not of chloroplasts, still showed the characteristic ntrc mutant level of lateral roots.Citation12 These results show that chloroplast function is required for the development of non-photosynthetic organs. Of course, this central role of the chloroplast is due to its function as source of nutrients derived of photosynthesis to feed the growth of heterotrophic tissues; however, the impairment of auxin synthesis in combination with the lateral root formation phenotype caused by NTRC deficiency, points to an important signaling function of the chloroplast. Chloroplast redox homeostasis might participate in signaling to harmonize the growth of photosynthetic and non-photosynthetic tissues, at least at early stages of seedling development.
| Abbreviations: | ||
| Fd | = | ferredoxin |
| FNR | = | ferredoxin-NADP oxidoreductase |
| FTR | = | ferredoxin-dependent thioredoxin reductase |
| NTRC | = | NADPH-dependent thioredoxin reductase C |
| Trx | = | thioredoxin |
Acknowledgments
This work was supported by ERDF-cofinanced grants from Ministry of Science and Innovation (BIO2010–15430) and Junta de Andalucía (BIO-182 and CVI-5919).
References
- Jacquot J-P, Eklund H, Rouhier N, Schürmann P. Structural and evolutionary aspects of thioredoxin reductases in photosynthetic organisms. Trends Plant Sci 2009; 14:336 - 43; http://dx.doi.org/10.1016/j.tplants.2009.03.005; PMID: 19446492
- Meyer Y, Reichheld JP, Vignols F. Thioredoxins in Arabidopsis and other plants. Photosynth Res 2005; 86:419 - 33; http://dx.doi.org/10.1007/s11120-005-5220-y; PMID: 16307307
- Serrato AJ, Pérez-Ruiz JM, Spínola MC, Cejudo FJ. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana.. J Biol Chem 2004; 279:43821 - 7; http://dx.doi.org/10.1074/jbc.M404696200; PMID: 15292215
- Pérez-Ruiz JM, Spínola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 2006; 18:2356 - 68; http://dx.doi.org/10.1105/tpc.106.041541; PMID: 16891402
- Moon JC, Jang HH, Chae HB, Lee JR, Lee SY, Jung YJ, et al. The C-type Arabidopsis thioredoxin reductase ANTR-C acts as an electron donor to 2-Cys peroxiredoxins in chloroplasts. Biochem Biophys Res Commun 2006; 348:478 - 84; http://dx.doi.org/10.1016/j.bbrc.2006.07.088; PMID: 16884685
- Pérez-Ruiz JM, Cejudo FJ. A proposed reaction mechanism for rice NADPH thioredoxin reductase C, an enzyme with protein disulfide reductase activity. FEBS Lett 2009; 583:1399 - 402; http://dx.doi.org/10.1016/j.febslet.2009.03.067; PMID: 19345687
- Spínola MC, Pérez-Ruiz JM, Pulido P, Kirchsteiger K, Guinea M, González MC, et al. NTRC new ways of using NADPH in the chloroplast. Physiol Plant 2008; 133:516 - 24; http://dx.doi.org/10.1111/j.1399-3054.2008.01088.x; PMID: 18346073
- Lepistö A, Kangasjärvi S, Luomala EM, Brader G, Sipari N, Keränen M, et al. Chloroplast NADPH-thioredoxin reductase interacts with photoperiodic development in Arabidopsis. Plant Physiol 2009; 149:1261 - 76; http://dx.doi.org/10.1104/pp.108.133777; PMID: 19151130
- Balmer Y, Vensel WH, Cai N, Manieri W, Schürmann P, Hurkman WJ, et al. A complete ferredoxin/thioredoxin system regulates fundamental processes in amyloplasts. Proc Natl Acad Sci U S A 2006; 103:2988 - 93; http://dx.doi.org/10.1073/pnas.0511040103; PMID: 16481623
- Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci U S A 2009; 106:9908 - 13; http://dx.doi.org/10.1073/pnas.0903559106; PMID: 19470473
- Kirchsteiger K, Ferrández J, Pascual MB, González M, Cejudo FJ. NADPH thioredoxin reductase C is localized in plastids of photosynthetic and nonphotosynthetic tissues and is involved in lateral root formation in Arabidopsis. Plant Cell 2012; 24:1534 - 48; PMID: 22505729
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 2002; 29:325 - 32; http://dx.doi.org/10.1046/j.0960-7412.2001.01217.x; PMID: 11844109