Abstract
Pollination drop (PD) secretion plays a critical role in wind pollination in many gymnosperms. We conducted detailed investigations on PD secretion in Ginkgo biloba, and found that PDs could not form when the micropyle was removed, but were able to form after removal of the shoot, leaves, ovular stalk, or ovular collar. The duration and volume of the PD increased under high relative humidity, but addition of salt or sugar did not affect PD secretion, its size, or its duration. Morphological and anatomical observations showed that many secretion cells at the nucellus tip contributed to secreting the PD after the formation of pollen chamber. Under laboratory conditions, the PD persisted for approximately 10 d if not pollinated, and re-formed five times after it was removed, with the total volume of PDs reaching approximately 0.4 μL. These results suggested that PDs can be continuously secreted by the tip of the nucellus cells during the pollination stage to increase the chance of capturing pollen from the air. Importantly, PD secretion is an independent behavior of the ovule and PDs were produced apoplastically.
Introduction
The majority of living gymnosperms are anemophilous (wind pollinated); that is, the airborne pollen is carried to the ovule by the wind. Although wind pollination is considered to be a random and metabolically wasteful process, the ovule has some pollination mechanisms that increase the chances of successful pollination. Among these mechanisms is the formation of a pollination drop (PD), which is secreted and extruded from the micropyle to capture pollen and transport it into the ovule.
PDs are known from primitive plants as early as the Carboniferous.Citation1 The PD also occurs widely in gymnosperms, including the Pinaceae, Taxodiaceae, Cupressaceae, Podocarpaceae, Taxaceae, and Cephalotaxaceae. It is also present in groups that are considered to be basal to conifers, such as cycads and Ginkgo.Citation2 The role of the PD in the pollination mechanism has been studied in several conifer families. For example, in western pine (Pinus monticola), a PD is secreted from the ovule, and it increases in size to fill the space between the micropylar arms. The drops scavenge pollen from the arms and nearby cone surfaces.Citation3 In yellow cypress (Chamaecyparis nootkatensis), each ovule exudes and withdraws a PD two to four times before the PD is permanently withdrawn.Citation4 In addition, a relationship between production of the PD and ovule orientation has been reported in conifers.Citation2 In general, most species with saccate pollen appear to produce PDs and have inverted ovules. The pollen trapped by the PD floats upward within the drop through the micropyle toward the nucellar tip,Citation5 and pollen flotation in the PD is an effective mechanism of pollen capture and transport in gymnosperms.Citation6 Many species with nonsaccate pollen also produce a PD, although their ovules generally do not show a favored orientation. Therefore, the pollen enters the drop and sinks into the ovule as the PD recedes down the micropyle canal.Citation7 These previous studies showed that the PD plays a key role in capturing pollen and transporting it to the ovule during the pollination process. However, there are many possibilities regarding the origins of the PD. Some studies reported that the PD is secreted from the nucellus because the nucellar tip closely resembles certain types of angiosperm nectaries in terms of its ontogeny, structure, and secretory products.Citation8,Citation9 However, other researchers suggested that the integument and megagametophyte also participate in the secretion process.Citation2 Furthermore, the PD can be augmented by rainwater or dew.Citation2,Citation10
Ginkgo is one of the oldest lineages of gymnosperms. The group once flourished in the early Mesozoic, but its main habitat was destroyed during later glaciations, and only one species, Ginkgo biloba L. now survives in China. Therefore, it is regarded as a “living fossil.” It is a dioecious, wind-pollinated gymnosperm, and more importantly, it shows highly efficient and successful pollination.Citation11 It has non-saccate pollen and a more or less erect ovule during pollination. The Ginkgo pollen chamber has been well-studied in previous anatomical work.Citation12,Citation13 Its nucellar cells may undergo programmed cell death to form a pollen chamber where pollen grains germinate and develop pollen-tubes.Citation14 The characteristic PD appears on the micropyle during the pollination stage,Citation15 and PD withdrawal behaviors are varied when triggered by different foreign pollen grains among taxa.Citation16 These results suggested that the PD may play an important role in capturing and facilitating pollen delivery into the ovule in G. biloba. To date, however, the mechanism by which the PD forms and the regulatory processes associated with it remain unclear.
In this paper, we analyzed the origins of the PD in G. biloba, and observed the patterns of secretion. The objective of this study was to investigate the behaviors of PD secretion in G. biloba and to unravel how the PD increases pollination efficiency. These findings increase our understanding of the pollination mechanisms in G. biloba.
Results
Ovule morphology and anatomical structure during pollination
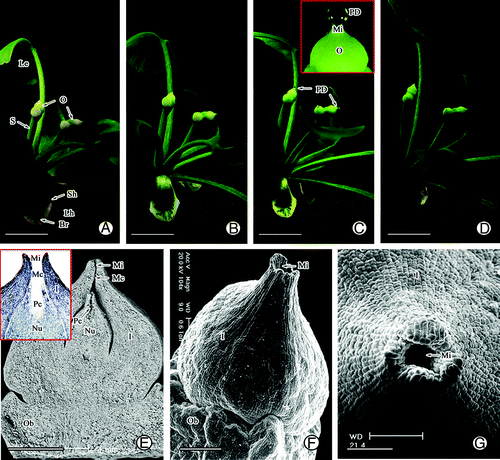
During pollination, there were 2–4 pairs of ovule tufts with 5–7 fan-shaped leaves spirally arranged at the apex of the short shoot (). In general, two ovules were located at the top of the stalk, and each ovule consisted of a slender stalk, ovule collar, integument, and nucellus before pollination (). Observation of the anatomical structure of the ovules showed that they had already differentiated into several structures including the micropyle, micropyle canal, and pollen chamber before pollination (). Among these structures, the micropyle, which was surrounded by the elongating outer integument tip, was funnel-shaped and opened upwards (). The outer surface of the apex integument was covered by wax (). The PD was secreted from the ovules and gradually formed on the micropyle during the pollination period (). The PD was spherical with a diameter range of 400–600 μm, about 2–3 times larger than that of the micropyle (). Interestingly, the ovule changed from yellow to green during formation of the PD ().
Figure 1. Morphology and structure of ovule in G. biloba during pollination stage. (A, B) Morphology of ovule before pollination. (C) PD secretion during pollination. (D) Morphology of ovule after pollination. (E) Anatomical structure of ovule. (F, G) Morphology of micropyle. Br, bract; I, integument; Le, leaf; Lh, long shoot; Mc, micropyle canal; Mi, micropyle; Nu, nucellus; O, ovule; Ob, ovule bracket; Pc, pollen chamber; PD, pollination drop; Sh, short shoot; S, stalk. Scale bars (A–D) = 1 cm, (E) = 1 mm, (F, G) = 200 μm.
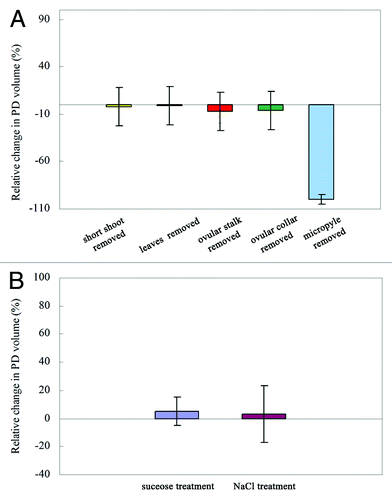
Influence of other organs and tissues on PD secretion
The ovule has a strong capacity to secrete PDs during pollination. To determine whether the other tissues and organs played roles in PD secretion, we investigated the effect of removing the short shoots, leaves, ovular stalk, ovular collars, and micropyle on PD secretion. The results showed removal of the short shoots, leaves, ovular stalk, and ovular collars from the ovule did not prevent formation of the PD (). In other words, the ovules can secrete PDs independently without the ovular collars and other tissues. However, when the micropyle of the ovule was partially removed, there was no PD formation ().
Influence of PD solute concentration on PD secretion
Next, we investigated the effects of solute pressure on PD secretion. We added extra sugar (sucrose) and salt (NaCl) into the PD on the living ovule as soon as the PD was produced. Untreated PDs served as the control. We observed the changes in PD volume under a stereomicroscope. The sugar and salt dissolved immediately into the PD; however, there were no detectable changes in PD volume after addition of sugar or salt, compared with the control PDs (). This indicated that the PD secretion behavior of the ovule was not affected by sugar and ion solute pressure in the PD.
PD secretion
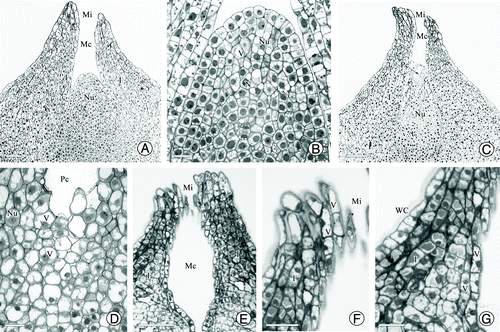
Before pollination, the nucellar tissue was cylindrical along the longitudinal section and its tip cells protruded upward (). Almost all nucellar cells were rich in cytoplasmic materials and contained vacuoles of various sizes that were scattered in the cytoplasm. The nuclei in nucellar cells and epidermis cells were prominent, and epidermal cells showed an orderly and compact arrangement (). The integument cells close to the micropyle canal also contained many large vacuoles (). As the pollination time approached, some nucellar tip cells became elongated and collapsed, forming a cavity surrounded by intact epidermal cells (). Then, the apical epidermal cells ruptured, resulting in an opening in the pollen chamber connecting to the micropyle canal (small insert figure in ). However, the nucellar cells below the pollen chamber still contained many vacuoles of different sizes and some cell walls appeared shriveled (). Observations of serial sections showed that the micropyle canal had an elliptical shape; that is, wide in the middle, but very thin at the top and bottom (). The cells of the micropyle were elongated and contained many vacuoles (). The integument around the micropyle canal consisted of 5–9 layers of small, closely arranged cells. The 2–3 layers of cells near the micropyle canal also contained many vacuoles ().
Figure 3. Structure of the nucellar apex and micropyle cells in G. biloba. (A) Ovule shape before pollination; (B) Nucellar cells before pollination; (C) Collapse of nucellar tip cells (arrow); (D) Nucellar cells below pollen chamber; (E) Morphology of micropyle canal; (F) Cells of micropyle; (G) Cells of micropyle canal. I, integument; Mc, micropyle canal; Nu, nucellus; Pc, pollen chamber; V, vacuole. Scale bars (A, C, E) = 100 μm; (B, D, F, G) = 50 μm.
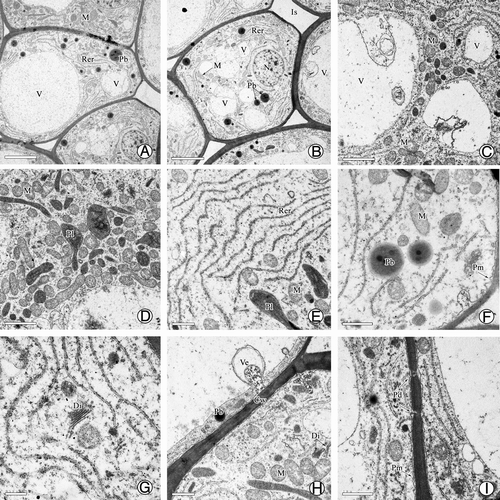
Ultrastructural observations showed that during PD production, most cell walls became thin and partially transparent, and each cell was filled with a large vacuole and abundant organelles such as mitochondria, rough endoplasmic reticulum (RER), plastids, and dictyosomes (). Importantly, a large number of mitochondria were distributed in the cytoplasm (), indicating that high energy metabolism was occurring in these secretion cells. In addition, the RER was evident and active, similar to mitochondria; there were abundant RER parallel to the cell wall in the cytoplasm and many protein bodies were located among them (). At the end of the RER, many vesicles formed and gradually merged into a large vesicle (). Afterwards, the vesicles were carried to the edge of cells to fuse with the plasma membrane, releasing their inclusions by exocytosis (). In addition, dictyosomes and many plastids were also distributed in these cells (), and plasmodesmata, which played an important role in transporting the secretion, were visible on the cell wall (). Together, these results demonstrated that PDs in G. biloba were produced by the nucellar cells after the formation of the pollen chamber, and some cells around the micropyle canal also played roles in the secretion. The secretion fluid was exuded into the micropyle canal, and finally formed the PD on the micropyle after filling the micropyle canal.
Figure 4. Ultrastructure of nucellar apex cells in G. biloba. (A, B) Nucellar apex cells; (C) Vacuome consisting of variously sized vacuoles; (D–G) Dense cytoplasm with abundant mitochondria, endoplasmic reticulum, protein bodies, and dictyosomes; (H) Vesicles near cell wall; (I) Plasmodesma connecting protoplasts of nucellar cells. Cw, cell wall; Di, dictyosome; Is, intercellular spaces; M, mitochondria; N, nucleus; Nu, nucellus; Pb, protein body; Pd, plasmodesma; Pl, plastid; Pm, plasma membrane; Rer, rough endoplasmic reticulum; V, vacuole; Ve, vesicle. Scale bars (A, B) = 5 μm; (C, D) = 2 μm; (E, F, G, I) = 1 μm; H = 0.5 μm.
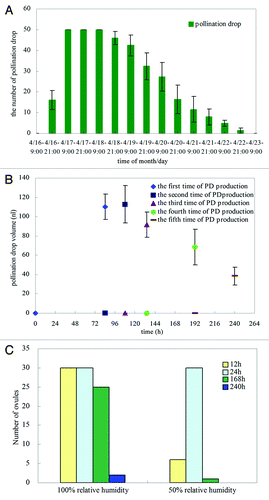
Characteristics of PD formation on the micropyle
PDs can be observed from early to late April in trees in the field, a period of approximately 20 d. However, it is very difficult to observe the PD production process in the field, so we performed these experiments under laboratory conditions (25°C and 50–70% relative humidity). The results showed that if artificial pollination was not performed, the formation of PDs usually started at approximately 21:00 h; the PDs accumulated and reached a peak at about 03:00–04:00 h; and the ovules were able to produce PDs for approximately 2 d (). The mean volume of the PD on the first day was approximately 25 nL, and the PDs were not reabsorbed the following morning. Generally, the volume of non-pollinated drops reached a maximum after approximately 84 h, and they retained their maximum volume for approximately 70 h. In addition, the non-pollinated PDs were able to be reproduced, and reached their maximum size at approximately 20 h after they were removed from the micropyle when they were at their original maximum size. Repeated production of PDs occurred five times in total, and the first two drops were the largest, each approximately 600 μm in diameter (volume of approx. 100 nL). The last three drops showed decreasing diameters, from approximately 400 to 200 μm, but these drops formed on the micropyle for longer than the first two drops. After five PDs had been produced, the ovule was unable to reproduce any more PDs (). Each ovule produced 0.4 μL of PDs in total (summing the volume of five PDs). Normally, the non-pollinated drops could persist for up to 7 d. Moreover, the ovules took approximately 12 h to produce PDs at 100% relative humidity (RH), and the PDs completely disappeared within 240 h. At 50% RH, the ovules took approximately 24 h to form PDs, and the PDs disappeared within 168 h ().
Discussion
Megastrobilus orientation and morphology are vital features for wind pollination in gymnosperms,Citation2,Citation17 because they influence the aerodynamics of pollen-grain deposition. The orientation and morphology of the megastrobilus contribute to generating a complex system of air eddies and strongly affect the direction of pollen movement and pollen capture.Citation17,Citation18 For example, ovules are surrounded by a number of fertile bracts, and the micropyles of ovules have multiple shapes, such as hook-like, flask-shaped, or two-pronged (micropyle arms) in most conifers.Citation2,Citation3,Citation9,Citation19 Compared with morphology of female cones in these gymnosperms, Ginkgo ovules are naked, lack bracts, the funnel-shaped micropyle is more or less erect, and the naked ovules are surrounded by fan-shaped leaves. The results show that G. biloba differs from some other conifer in terms of ovular structure and orientation characteristics, and these features are clearly associated with some pollination characteristics during pollination.
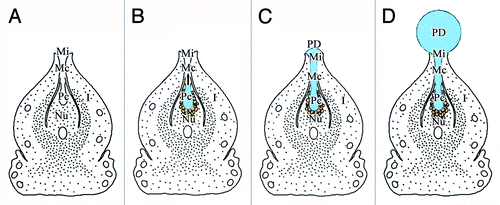
Pollination drops, secreted by the ovule, are involved in complex pollination mechanisms, and play important roles in the reproductive success of gymnosperms.Citation10 However, several hypotheses have been put forward regarding the origins of the PD. Anatomical studies suggest that the nucellus is the probable origin of the PD,Citation9,Citation20 but this is controversial, because the gymnosperm ovule is unvascularized and lacks conducting cells. The integument and megagametophyte have also been proposed as the origins of the PD.Citation10 Although the degeneration of the nucellus cells is consistent with PD production, the manner of degeneration differs among genera. For instance, in Cephalotaxus,Citation21 Cupressus,Citation22 and Podocarpus,Citation23 the breakdown products are transferred to the PD after degeneration of the nucellus cells, while only the uppermost layer of nucellus cells break down in SequoiadendronCitation19 and Phyllocladus.Citation22 Moreover, PDs are produced without nucellus degeneration in Cedrus,Citation9 Taxus,Citation24 Larix,Citation25 and Pseudotsuga.Citation26 In the present study, we found that many cells with typical secretion characteristics were located at the tip of the nucellus and inner integument during the pollination stage in Ginkgo. At this stage, the nucellar tissues had degenerated and broken down into the pollen chamber. In other words, the nucellus tissues had broken down before PD production. This finding suggested that the Ginkgo PD mainly originated from the nucellus; the integument cells in the micropyle were also involved in the PD secretion process. Thus, in the PD production process, the pollen chamber opening formed before PD production ( and ), and the PD was produced in, and initially accumulated in, the pollen chamber (). When the volume of the PD exceeded that of the pollen chamber, it began to flow into the micropyle canal through the pollen chamber opening (), and finally protruded from the micropyle (). Additionally, because of the funnel-like shape and the wax-layer covering of the micropyle, the continuous secretion of the PD resulted in the formation of a bead (). Finally, the PD became spherical and about double the size of the micropyle ( and ).
Figure 6. Pattern of PD production. (A) Formation of pollen chamber before PD production; (B) Collapse of nucellar apex cells during PD production; (C) Exudation of PD from pollen chamber to micropyle; (D) Constant production of PD by nucellar cells around pollen chamber, PD reaches maximum volume at pollination stage. I, integument; Mc, micropyle canal; Mi, micropyle; Nu, nucellus; Pc, pollen chamber; PD, pollination drop.
Secretion of the PD is also affected by the outside environment. For example, it is thought that the secretion is a type of guttation in Pinus.Citation27 High relative humidity is thought to be essential for PD formation in the Taxus, but rainfall can disrupt pollination as it washes away the PDs and decreases the rate of fertilization success.Citation10 In addition, PDs contain sugars (sucrose, glucose, and fructose) as the main components,Citation10,Citation28 and the concentrations of some ions differs among conifer speciesCitation28 indicating that sugars and inorganic ions are significant functional components of the PD. In this study, we found that the duration and volume of the PD were increased when ovules were kept under high relative humidity. We also found that the PD was secreted independently of the ovular collars, ovular stalk, and leaves, and that PD secretion behavior was not affected by sugar and ion solute pressure in the PD. These results indicated that the PD was produced apoplastically and that changes in the composition of the PD did not affect its secretion or duration. However, PD components are complex and PD secretion can be inhibited by its own pollen.Citation16 Thus, there might be some other materials that affect ovule secretion.
The duration of the PD under non-pollinated conditions varies among different gymnosperms. It has been suggested that the duration of the PD is largely determined by its cone or ovule,Citation29,Citation30 and ecological conditions.Citation3 For example, the PD can persist for several weeks in other species, e.g., Taxus brevifolia and Juniperus communis.Citation24,Citation31 In the Cupressaceae, Taxodiaceae, Cephalotaxaceae, and Taxaceae, it can be secreted at night but withdraw during daytime in a repeating cycle.Citation2 In our studies, the PD of G. biloba lasted for approximately 7 d under experimental conditions (25°C, approx. 70% relative humidity). In addition, the ovule showed a strong capacity to produce the PD during pollination. The volume of each PD was 100–120 nL, and the total volume of PDs produced by each ovule was 400 nL under non-pollinated conditions. In contrast, the volume of the PD reaches only 250 nL in the single-ovuled cone of Taxus baccata.Citation28 Under non-pollinated conditions the Ginkgo PD could be reproduced five times after it was removed, showing a prolonged period of PD production. These results suggested that the ovules could persistently and repeatedly secrete abundant PDs during the pollination period, which would not only increase the ability of the ovule to capture pollen from the air, but would also ensure that the ovules can replace PDs that fall as a result of wind action or evaporate in strong sunlight.
Materials and Methods
Plant material
Healthy female G. biloba trees were selected from the Ginkgo Experimental Station in Yangzhou University, Yangzhou, China (32°20′N, 119°30′E). Materials were collected from 30 plants of similar age (approx. Twenty years old) and height (approx. Four m) during the pollination period in early April from 2009 to 2012.
PD secretion
Branches with ovules that had just secreted a PD were collected and kept in the laboratory. Short shoots with 3–5 ovules were cut with loppers and inserted into a sponge that was immersed in a tray containing water. When the PD appeared, pollination secretion experiments were performed as follows: (1) Under laboratory conditions (Room temperature about 25°C and 50–70% relative humidity), PD secretion was observed at 1 h intervals under a stereomicroscope (n = 50 ovules). (2) Ovules were placed in incubators (25°C and about 70% relative humidity), the PD was removed after it reached its maximum volume, and we observed whether it was reproduced. Then, we calculated the total volume (n > 30). (3) Ovules were placed in incubators with 50% and 100% relative humidity to compare the effects of different relative humidities on PD formation; (3) Branches with ovules secreting PDs were kept in the laboratory. To determine which other tissues and organs were important for secretion of the PD, the short shoots, leaves, ovular stalk, ovular collars, and micropyle were removed separately, as follows: a) The short shoots and leaves were removed, leaving the ovules, ovule collar and ovule stalk intact; b) The ovule stalk was removed, leaving the ovule with the ovule collar intact; c) The ovule collar was removed, leaving the ovule; d) The two ovules at the end of one ovule stalk were removed together, then one ovule micropyle was removed and the other ovule micropyle was left intact. Then, the remaining parts of a), b), c) and d) were inserted into or on a sponge that was immersed in a tray containing water. All the PDs were removed with absorbent paper. We observed the tissues to determine whether the PD secretion was inhibited. (4) Since sugars and inorganic ions are significant functional components of the PD, we added sugar (sucrose) and salt (NaCl) powder into the PD secreted from the ovule, to determine whether the PD size and duration were affected by sugar and ion concentrations. Sugar and salt were each ground into a powder using a mortar. Then, a human eyelash was used to collect the powder sugar and salt, which was gently applied to the PD by touching it. Untreated PDs served as the control. We observed the PD to record changes in its size or duration.
PD volume
A stereomicroscope with micrometer eyepiece was used to measure the diameter of the PD at 1 h intervals from secretion to withdrawal (n > 30). The volume (V) of the PD, which was assumed to be spherical, was calculated using the equation V = (4/3) πr3, where r is the PD radius.
Scanning electron microscopy (SEM)
Samples were prepared for SEM observation as follows. Ovules were collected and fixed in an improved FAA solution (50% alcohol:glacial-acetic-acid:formaldehyde = 89:6:5) for 4 h at room temperature and then kept for 12 h at 4°C. Then, they were dehydrated in a graded ethanol series (30, 50, 70, 80, 90, and 100%, 15 min each step). After drying at critical point, the specimens were coated with a layer of gold, and observed under a Hitachi H-300 SEMCitation32
Semi-thin sections
The preparation of material for semi-thin section techniques was as follows. Ovules were collected during pollination and prefixed in 2.5% (v/v) glutaraldehyde plus 1.5% (w/v) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), for 20 h at room temperature. After being postfixed in 1% (w/v) osmium tetroxide for 6 h at 4°C, the samples were rinsed twice, and then dehydrated in an alcohol series (30, 50, 70, 80, 90, and 100%, 15 min each step). The samples were infiltrated and finally embedded in Spurr’s resin. Sections of 1 µm in thickness were successively cut with glass knives, and then stained with 1% (w/v) toluidine blue O dissolved in 1% (w/v) sodium borate prior to examination. The samples were observed under a microscope (Zeiss Axioskop 40, Carl Zeiss Shanghai Company Ltd., Shanghai, China) with digital images captured using an Axiocam MRC camera.Citation33
Transmission Electron Microscopy (TEM)
Samples were embedded in Spurr’s resin, as for the semi-thin sections. Sections of 70 nm in thickness were cut with a diamond knife, stained with 1% uranyl acetate, and 1% lead citrate. Secreting cells were examined and imaged under a Philips Tecnai 12 TEM.
Acknowledgments
We thank Prof. Peng Chen and Prof. Zhong Wang of Yangzhou University, China, for technical advice on the experiment, and Dr. Nianjun Teng of Nanjing Agricultural University for reviewing our manuscript. This work was supported by the Natural Science Foundation of Jiangsu Province (No. BK2011444) and the National Natural Science Foundation of China (No. 30870436).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ contributions
BJ and LW designed the project; XXJ, DW, and LZ participated in SEM and TEM observations and semi-thin sectioning experiments; LZ and YLW were involved in cultivating ovules and collecting and measuring pollination drops; BJ and LW drafted the manuscript and all co-authors participated in its editing.
References
- Rothwell GW. Evidence for a pollination-drop mechanism in paleozoic pteridosperms. Science 1977; 198:1251 - 2; http://dx.doi.org/10.1126/science.198.4323.1251; PMID: 17741704
- Owens JN, Takaso T, Runions CJ. Pollination in conifers. Trends Plant Sci 1998; 3:479 - 85; http://dx.doi.org/10.1016/S1360-1385(98)01337-5
- Owens JN, Gatalano G, Bennett JS. The pollination mechanism of western white pine. Can J For Res 2001; 31:1731 - 41; http://dx.doi.org/10.1139/x01-095
- Owens JN, Simpson S, Molder M. The pollination mechanism in yellow cypress (Chamaecyparis nootkatensis). Can J For Res 1980; 10:564 - 72; http://dx.doi.org/10.1139/x80-093
- Owens JN, Blake MD. The pollination mechanism of Sitka spruce (Picea sitchensis). Can J Bot 1984; 62:1136 - 48; http://dx.doi.org/10.1139/b84-158
- Leslie AB. Flotation preferentially selects saccate pollen during conifer pollination. New Phytol 2010; 188:273 - 9; http://dx.doi.org/10.1111/j.1469-8137.2010.03356.x; PMID: 20579290
- Tomlinson PB. Functional morphology of saccate pollen in conifers with special reference to Podocarpaceae. Int J Plant Sci 1994; 155:699 - 715; http://dx.doi.org/10.1086/297209
- Takaso T, Owens JN. Ovulate cone morphology and pollination in Pseudotsuga and Cedrus.. Int J Plant Sci 1995; 156:630 - 9; http://dx.doi.org/10.1086/297285
- Takaso T, Owens JN. Pollination drop and microdrop secretions in cedrus.. Int J Plant Sci 1995; 156:640 - 9; http://dx.doi.org/10.1086/297286
- Gelbart G, von Aderkas P. Ovular secretions as part of pollination mechanisms in conifers. Ann For Sci 2002; 59:345 - 57; http://dx.doi.org/10.1051/forest:2002011
- Wang L, Jin B, Lu Y, Chen P. Research progress in pollination biology of Ginkgo biloba L. Acta Bot Boreal Occident Sin 2009; 29: 0842–0850.
- De Sloover-Colinet A. Chamber pollinique et gametophyte male chez Ginkgo biloba.. Cellule 1963; 64:129 - 45
- Friedman WE. Growth and development of the male gametophyte of Ginkgo biloba within the ovule (in vivo). Am J Bot 1987; 74:1797 - 815; http://dx.doi.org/10.2307/2443963
- Li DH, Yang X, Cui KM, Li ZL. Morphological changes in nucellar cells undergoing programmed cell death (PCD) during pollen chamber formation in Ginkgo biloba.. Acta Bot Sin 2003; 45:53 - 63
- Wang L, Jin B, Lu Y, Jin XX, Teng NJ, Chen P. Developmental characteristics of the ovule and its biological significance in Ginkgo biloba L. J Beijing Forestry University 2010; 32: 29–85.
- Jin B, Zhang L, Lu Y, Wang D, Jiang XX, Zhang M, et al. The mechanism of pollination drop withdrawal in Ginkgo biloba L. BMC Plant Biol 2012; 12:59; http://dx.doi.org/10.1186/1471-2229-12-59; PMID: 22548734
- Schwendemann AB, Wang G, Mertz ML, McWilliams RT, Thatcher SL, Osborn JM. Aerodynamics of saccate pollen and its implications for wind pollination. Am J Bot 2007; 94:1371 - 81; http://dx.doi.org/10.3732/ajb.94.8.1371; PMID: 21636505
- Niklas KJ. The aerodynamics of wind pollination. Bot Rev 1985; 51:328 - 87; http://dx.doi.org/10.1007/BF02861079
- Takaso T, Owens JN. Ovulate cone, pollination drop, and pollen capture in Sequoiadendron (Taxodiaceae). Am J Bot 1996; 83:1175 - 80; http://dx.doi.org/10.2307/2446201
- Xing SP, Chen ZK, Hu YX, Zhou F, Lin JX. Ovule development, formation of pollination drop and pollination process in Taxus chinensis (Taxaceae). Acta Bot Sin 2000; 42:126 - 32
- Chesnoy L. Les sécrétions dans la pollinisation des Gymnospermes. Acta Bot Gallica 1993; 140:145 - 56
- Tison A. Remarques sur les gouttelettes collectrices des ovules des coniferes. Memoires de la Societe Linneene de Normandie 1911; 23:51 - 64
- Tomlinson PB, Braggins JE, Rattenbury JA. Pollination drop in relation to cone morphology in Podocarpaceae: a novel reproductive mechanism. Am J Bot 1991; 78:1289 - 303; http://dx.doi.org/10.2307/2444932
- Anderson ED, Owens JN. Microsporogenesis, pollination, pollen germination and male gametophyte development in Taxus brevifolia.. Ann Bot (Lond) 2000; 86:1033 - 42; http://dx.doi.org/10.1006/anbo.2000.1274
- Owens JN, Morris S, Catalano G. How the pollination mechanism and prezygotic and postzygotic events affect seed production in Larix occidentalis.. Can J For Res 1994; 24:917 - 27; http://dx.doi.org/10.1139/x94-121
- Takaso T, Owens JN. Postpollination-prezygotic ovular secretions into the micropylar canal in Pseudotsuga menziesii (Pinaceae). J Plant Res 1996; 109:147 - 60; http://dx.doi.org/10.1007/BF02344540
- McWilliam JR. The role of the micropyle in the pollination of Pinus.. Bot Gaz 1958; 120:109 - 17; http://dx.doi.org/10.1086/336010
- Nepi M, von Aderkas P, Wagner R, Mugnaini S, Coulter A, Pacini E. Nectar and pollination drops: how different are they?. Ann Bot 2009; 104:205 - 19; http://dx.doi.org/10.1093/aob/mcp124; PMID: 19477895
- von Aderkas P, Leary C. Micropyle exudates in Douglas fir–timing and volume of production. Sex Plant Reprod 1999; 11:354 - 6; http://dx.doi.org/10.1007/s004970050163
- O’Leary SJB, von Aderkas P. Postpollination drop production in hybrid larch is not related to the diurnal pattern of xylem water potential. Trees (Berl) 2006; 20:61 - 6; http://dx.doi.org/10.1007/s00468-005-0013-7
- Mugnaini S, Nepi M, Guarnieri M, Piotto B, Pacini E. The pollination mechanism in Juniperus communis: response to deposited material. Ann Bot (Lond) 2007; 100:1475 - 81; http://dx.doi.org/10.1093/aob/mcm253
- Jin B, Wang L, Wang J, Teng NJ, He XD, Mu XJ, et al. The structure and roles of sterile flowers in Viburnum macrocephalum f. keteleeri (Adoxaceae). Plant Biol (Stuttg) 2010; 12:853 - 62; http://dx.doi.org/10.1111/j.1438-8677.2009.00298.x; PMID: 21040300
- Jin B, Wang L, Wang J, Jiang KZ, Wang Y, Jiang XX, et al. The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana.. BMC Plant Biol 2011; 11:35; http://dx.doi.org/10.1186/1471-2229-11-35; PMID: 21329528