Abstract
The rolB oncogene was previously identified as an important player in ROS metabolism in transformed plant cells. Numerous reports indicate a crucial role for animal oncogenes in apoptotic cell death. Whether plant oncogenes such as rolB can induce programmed cell death (PCD) in transformed plant cells is of particular importance. In this investigation, we used a single-cell assay based on confocal microscopy and fluorescent dyes capable of discriminating between apoptotic and necrotic cells. Our results indicate that the expression of rolB in plant cells was sufficient to decrease the proportion of apoptotic cells in steady-state conditions and diminish the rate of apoptotic cells during induced PCD. These data suggest that plant oncogenes, like animal oncogenes, may be involved in the processes mediating PCD.
The interplay between oncogene expression, ROS production and apoptosis has received a great deal of attention in animal cell biology. Few studies have focused on the role of plant oncogenes in these processes. Plant oncogenes are Agrobacterium T-DNA genes of the plast (RolB) family of eukaryotic regulators.Citation1 During plant infection by the pathogen Agrobacterium rhizogenes, the rolA, rolB and rolC oncogenes are transferred from the bacteria into the plant genome, causing tumor formation and hairy root disease.Citation2 The function of rolB is not restricted to root formation; the gene promotes de novo formation of floral and shoot meristems, induces parthenocarpy, causes a delay in pistil and anther development and modifies the balance between the proliferation of procambial cells and xylem differentiation.Citation3 The mechanism by which the RolB oncoprotein exerts such varied morphological changes remains unknown.
Recently, involvement of the rolB and rolC plant oncogenes in ROS metabolism was revealed.Citation4,Citation5 Both genes, when expressed in plant cells, decreased intracellular ROS level. The rolB gene acts by inducing upregulation of antioxidant genes, thereby permanently supporting an active anti-oxidative status of transformed cells.Citation5 As ROS perturbations and apoptotic processes are tightly linked, we proposed involvement of the RolB protein in signaling networks regulating PCD in plants. The present investigation was aimed at testing this possibility.
Necrosis, a rapid form of cell death, is characterized by cytoplasmic swelling, destruction of organelles and disruption of the plasma membrane.Citation6 Apoptosis is characterized by cell shrinkage, maintenance of organelle integrity and condensation and fragmentation of DNA.Citation6 A distinct type of PCD called apoptosis was originally identi□ed in single cells that were usually surrounded by healthy-looking neighbors.Citation6 In the present work, we used the terms “PCD” and “apoptosis” interchangeably. Early symptoms of apoptosis, such as nuclear chromatin condensation at the periphery of nucleus and around nucleoli, were studied. To distinguish between necrotic and apoptotic types of cell death, we used confocal microscopy and differential staining with fluorescent dyes.
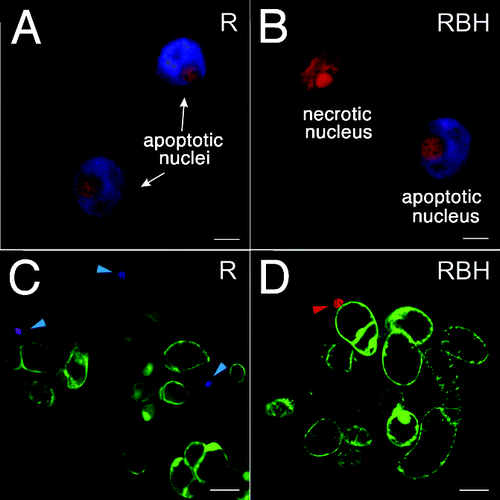
Experiments were performed with Rubia cordifolia cell suspension cultures. The control (non-transformed) cell line R was transformed with the rolB gene to generate cell lines RBM and RBH with different level of the target gene expression.Citation5 The strength of expression of rolB in the RBM culture is similar to the expression of rolB from its own 5′ promoter.Citation7 In the RBH culture, rolB has a 2.5-fold higher expression level than in the RBM culture. Thus, RBH represents a culture with the maximum possible rolB transcript abundance; increasing the expression of rolB in R. cordifolia cells to higher levels induces cell death.Citation5,Citation7 The stability of the rolB gene expression was controlled during this work. The cell suspension cultures were grown for 4–5 d (late exponential stage) and analyzed by confocal microscopy. The cells were stained with Hoechst 33342 and propidium iodide. Hoechst 33342 was used to visualize nuclei undergoing fragmentation during apoptosis and the subsequent formation of apoptotic bodies (Fig. One A, B). Hoechst 33342 marks early stages of PCD prior to the disappearance of dead cells and serves as a good marker for apoptosis both in plant and animal cells.Citation8 Propidium iodide can only enter cells with damaged membranes; inside cells, it intercalates into double-stranded nucleic acids, resulting in a bright red fluorescence in non-viable cells, particularly in the nucleus (Fig. One B, see the top, red-colored nucleus). Apoptotic nuclei in the R and rolB-expressing cells had a similar morphology as demonstrated in (compare apoptotic nuclei in A and B panels). A view of R. cordifolia cells triple-stained with fluorescein diacetate, propidium iodide and Hoechst 33342 is presented in , panels C and D. Living cells were detected by fluorescein diacetate (green staining). In the non-transformed cells (C), blue staining of the nuclei is shown by cyan arrows. Cells containing these nuclei are in an early phase of PCD. A later phase of PCD is characterized by violet-stained nuclei (Fig. One C). In rolB-expressing cells, apoptotic cells were rarely detected (compare C and D panels). A necrotic cell of the RBH culture with a red-stained nucleus is indicated by a red arrow (Fig. One D).
Figure 1. Laser confocal microscopy of R. cordifolia cells. Cells of the non-transformed R culture (A), (C) and rolB-transformed RBH culture (B), (D) were double-stained with propidium iodide and Hoechst 33342 as described in the Materials and Methods. (A), (B) Nuclei of R. cordifolia cells dyed to indicate necrosis (red) and apoptosis (blue + red) are presented. Propidium iodide was used to detect collapsed nuclei of necrotic cells. Hoechst 33342 was used to detect features of apoptotic cells, i.e., chromatin condensation at the periphery of nucleus and around nucleoli. (C), (D) A view of R. cordifolia cells triple-stained with fluorescein diacetate, propidium iodide and Hoechst 33342. Living cells were visualized with fluorescein diacetate (green cells). In a sample of control non-transformed cells (C), blue staining of the nuclei (cyan arrows) indicates an early phase of PCD and violet staining of the nucleus reflects a later phase of PCD. A necrotic cell with a red-stained nucleus is indicated by red arrow. Scale bars, 5 µm (A), (B) and 50 µm (C), (D).
In steady-state (resting) conditions, the control R culture contained both necrotic and apoptotic cells (). The frequency of necrotic cells in the rolB-expressing line was similar to that in the control (). At the same time, the percentage of apoptotic cells in the rolB-expressing cells was significantly lower than in the control cells. The maximal effect was found in the RBH cell culture, which contained three times fewer apoptotic cells than the control (0.8% in the RBH culture vs. 2.3% in the R culture, ).
Table 1. Percentage of necrotic and apoptotic cells in the total cell pool of R. cordifolia cell suspension cultures
To induce PCD, we used known inducers of PCD, such as menadione, 6-benzylaminopurine and methyl jasmonate.Citation9-Citation11 The latter substance did not increase the frequency of apoptotic cells in the control cells and therefore was not included in the analysis. Menadione and 6-benzylaminopurine showed a clear induction of PCD in the R cells (). Using this background, we could detect a substantial inhibition of PCD symptoms in the RBM and RBH cultures (). In high-rolB-expressing cells, almost no response was detected for menadione- and 6-benzylaminopurine-induced PCD, while in R line, the percentage of PCD-damaged cells was substantially increased. This indicates that rolB attenuates or even prevents induced PCD. Similar results were obtained with cultures in stationary phase (data not shown).
Although plants contain several animal protooncogene homologs, such as c-myb, c-myc, c-fos, c-jun and ras,Citation12 they contain no obvious homologs of crucial animal regulators of apoptosis (Bcl-2 family proteins, caspases, Ced-4, p53 and NF-κB).Citation13 Nevertheless, introduction of mammalian death regulators in yeast and plant cells induced the expected phenotype with regard to the nature of the gene introduced. This observation supports broad evolutionary conservation and functional similarity of apoptotic processes in eukaryotic organisms.Citation14-Citation16 There may be a functional parallel between the RolB protein family and animal Bcl-2 protein family members, namely pro-survival proteins such as BCL-2 and BCL-XL, which repress ROS-mediated programmed cell death.Citation17
The role of excessive ROS in plant PCD is well established.Citation18 The mechanism by which rolB inhibits PCD in transformed culture cells is likely by upregulation of antioxidant genes. It was previously shown that the majority of antioxidant genes studied, including those encoding the Cu/Zn superoxide dismutases, catalases, ascorbate peroxidases and class III peroxidase genes, were coordinately upregulated in rolB-expressing cells.Citation5,Citation19 RolB permanently sustains an active anti-oxidative environment in transformed cells and simultaneously maintains their normal redox balance, providing physiological conditions for adaptation to external oxidative stress.Citation5 While the cellular conditions leading to PCD induction are linked with an imbalance in redox status,Citation20 this function of RolB may be important for PCD prevention.
The rolB gene also attenuated necrotic cell death caused by menadione and 6-benzylaminopurine (). This result confirms our previous observations indicating that rolB inhibited necrotic cell death by preventing ROS elevation induced by menadione.Citation5 6-Benzylaminopurine does not induce excessive ROS, and its mechanism of necrotic/pro-apoptotic action is not known.Citation10 Therefore, it seems likely that rolB also prevents ROS-independent cell death. The results presented in this work indicate that plant oncogenes may be involved in processes regulating PCD.
Materials and Methods
Cell suspension cultures
Cell suspension cultures, such as R (non-transformed control), RBM (cell line with a medium level of rolB expression) and RBH (cell line with a high level of rolB expression) of R. cordifolia L. (Rubiaceae), were described previously.Citation5 Cell suspensions were cultivated using liquid WB/A medium supplemented with 0.5 mg/l 6-benzylaminopurine and 2.0 mg/l α-naphthaleneacetic acid in the dark at 24°C with 12-d subculture intervals.Citation5
Laser confocal microscopy
To monitor PCD, we used a standard protocol from Molecular Probes based on confocal microscopy and fluorescent dyes Hoechst 33342 and propidium iodide. Suspensions of plant cells were grown in liquid WB/A medium for 4–12 d and filtered through 100-μm mesh nylon to separate cell clusters. Single cells and cell aggregates were gently centrifuged and re-suspended in liquid WB/A medium containing 5 μg/ml bisbenzimide (Hoechst 33342, Molecular Probes, Eugene, OR) and 5 µg/ml propidium iodide (Molecular Probes) and incubated at 25 ± 1°C in the dark. Cells were incubated with the dyes for 10 min. Dye-loaded cells were washed with the WB/A medium and re-suspended. Apoptotic nuclei were visualized by staining with Hoechst 33342. Propidium iodide was used to detect dead cells. In some experiments, triple staining was used by adding 10 μg/ml fluorescein diacetate (Sigma Aldrich Chem. Corp., USA) to visualize living cells. Examination of fluorescence in single cells was performed with a LSM 510 META confocal laser scanning microscope (Carl Zeiss, Germany) and LSM 710 LIVE confocal microscope (Carl Zeiss) in the Far Eastern Instrumental Centre for Biotechnology and Gene Engineering (Institute of Biology and Soil Science). For excitation of dyes, laser lines 405, 488 and 543 nm and the corresponding emission filters were used to detect Hoechst 33342, fluorescein diacetate and propidium iodide, respectively . Video files of the captured images were recorded and analyzed with LSM 510 Release 3.5 and ZEN 2010 software. Quantitative experiments were repeated two to three times. In total, approximately 1000 cells were counted for each treatment.
Menadione and 6-benzylaminopurine treatments
Menadione (Sigma Aldrich) was added to 4-d suspension cultures of R. cordifolia, which were subsequently cultivated for 3 h. Four-day suspension cultures were cultivated in the presence of 6-benzylaminopurine (Sigma Aldrich, Plant Tissue Culture Grade) for 4 d and then were analyzed. Different concentrations of menadione and 6-benzylaminopurine were previously tested to find suitable concentrations for R. cordifolia cells. The doses 100 μM of menadione and 60 μM of 6-benzylaminopurine were found to be adequate to test PCD symptoms in control and transformed cells.
| Abbreviations: | ||
| PCD | = | programmed cell death |
| ROS | = | reactive oxygen species |
Acknowledgments
This work was supported by a grant from the Russian Foundation for Basic Research and by the Grant Program “Molecular and Cell Biology” of the Russian Academy of Sciences.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Levesque H, Delepelaire P, Rousé P, Slightom J, Tepfer D. Common evolutionary origin of the central portions of the Ri TL-DNA of Agrobacterium rhizogenes and the Ti T-DNAs of Agrobacterium tumefaciens.. Plant Mol Biol 1988; 11:731 - 44; http://dx.doi.org/10.1007/BF00019514
- Spena A, Schmülling T, Koncz C, Schell JS. Independent and synergistic activity of rol A, B and C loci in stimulating abnormal growth in plants. EMBO J 1987; 6:3891 - 9; PMID: 16453816
- Cecchetti V, Altamura MM, Serino G, Pomponi M, Falasca G, Costantino P, et al. ROX1, a gene induced by rolB, is involved in procambial cell proliferation and xylem differentiation in tobacco stamen. Plant J 2007; 49:27 - 37; http://dx.doi.org/10.1111/j.1365-313X.2006.02934.x; PMID: 17233794
- Bulgakov VP, Aminin DL, Shkryl YN, Gorpenchenko TY, Veremeichik GN, Dmitrenok PS, et al. Suppression of reactive oxygen species and enhanced stress tolerance in Rubia cordifolia cells expressing the rolC oncogene. Mol Plant Microbe Interact 2008; 21:1561 - 70; http://dx.doi.org/10.1094/MPMI-21-12-1561; PMID: 18986252
- Bulgakov VP, Gorpenchenko TY, Veremeichik GN, Shkryl YN, Tchernoded GK, Bulgakov DV, et al. The rolB gene suppresses reactive oxygen species in transformed plant cells through the sustained activation of antioxidant defense. Plant Physiol 2012; 158:1371 - 81; http://dx.doi.org/10.1104/pp.111.191494; PMID: 22271748
- Assunção Guimarães C, Linden R. Programmed cell deaths. Apoptosis and alternative deathstyles. Eur J Biochem 2004; 271:1638 - 50; http://dx.doi.org/10.1111/j.1432-1033.2004.04084.x; PMID: 15096203
- Shkryl YN, Veremeichik GN, Bulgakov VP, Tchernoded GK, Mischenko NP, Fedoreyev SA, et al. Individual and combined effects of the rolA, B, and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol Bioeng 2008; 100:118 - 25; http://dx.doi.org/10.1002/bit.21727; PMID: 18023060
- Elbaz M, Avni A, Weil M. Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death Differ 2002; 9:726 - 33; http://dx.doi.org/10.1038/sj.cdd.4401030; PMID: 12058273
- Sun Y, Zhou J, Dai Y, Zhai Z. Menadione-induced apoptosis and its mechanism in plants. Chin Sci Bull 2000; 45:350 - 4; http://dx.doi.org/10.1007/BF02909767
- Carimi F, Zottini M, Formentin E, Terzi M, Lo Schiavo F. Cytokinins: new apoptotic inducers in plants. Planta 2003; 216:413 - 21; PMID: 12520332
- Repka V. Hydrogen peroxide generated via the octadecanoid pathway is neither necessary nor sufficient for methyl jasmonate-induced hypersensitive cell death in woody plants. Biol Plant 2002; 45:105 - 15; http://dx.doi.org/10.1023/A:1015112926955
- Loidl A, Loidl P. Oncogene- and tumor-suppressor gene-related proteins in plants and fungi. Crit Rev Oncog 1996; 7:49 - 64; PMID: 9109497
- Kawai-Yamada M, Ohori Y, Uchimiya H. Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell 2004; 16:21 - 32; http://dx.doi.org/10.1105/tpc.014613; PMID: 14671021
- Lacomme C, Santa Cruz S. Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc Natl Acad Sci U S A 1999; 96:7956 - 61; http://dx.doi.org/10.1073/pnas.96.14.7956; PMID: 10393929
- Kawai-Yamada M, Jin L, Yoshinaga K, Hirata A, Uchimiya H. Mammalian Bax-induced plant cell death can be down-regulated by overexpression of Arabidopsis Bax Inhibitor-1 (AtBI-1). Proc Natl Acad Sci U S A 2001; 98:12295 - 300; http://dx.doi.org/10.1073/pnas.211423998; PMID: 11593047
- Chen SR, Dunigan DD, Dickman MB. Bcl-2 family members inhibit oxidative stress-induced programmed cell death in Saccharomyces cerevisiae.. Free Radic Biol Med 2003; 34:1315 - 25; http://dx.doi.org/10.1016/S0891-5849(03)00146-1; PMID: 12726919
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9:47 - 59; http://dx.doi.org/10.1038/nrm2308; PMID: 18097445
- Kawai-Yamada M, Yoshinaga K, Ogawa T, Ihara-Ohori Y, Uchimiya H. Oxidative stress and plant cell death suppressors. Plant Biotechnol 2005; 22:419 - 22; http://dx.doi.org/10.5511/plantbiotechnology.22.419
- Veremeichik GN, Shkryl YN, Bulgakov VP, Avramenko TV, Zhuravlev YN. Molecular cloning and characterization of seven class III peroxidases induced by overexpression of the agrobacterial rolB gene in Rubia cordifolia transgenic callus cultures. Plant Cell Rep 2012; 31:1009 - 19; http://dx.doi.org/10.1007/s00299-011-1219-3; PMID: 22238062
- de Pinto MC, Tommasi F, De Gara L. Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiol 2002; 130:698 - 708; http://dx.doi.org/10.1104/pp.005629; PMID: 12376637