Abstract
In a previous study, we demonstrated that Arabidopsis Antioxidant Protein1 (ATX1) plays an essential role in copper (Cu) homeostasis, conferring tolerance to both excess and subclinically deficient Cu. The Cu-binding motif MXCXXC was required for the physiological function of ATX1. In this study, we found that overexpression of ATX1 resulted in hypersensitivity to severe Cu deficiency despite enhancing tolerance to subclinical Cu deficiency. However, overexpression of mutated ATX1, replacing the Cu-binding motif MXCXXC with MXGXXG, abolished the hypersensitivity, for no differences from the wild type under the same conditions. Thus, the expression of ATX1 must be cautiously regulated to avoid homeostatic imbalance with the over-chelation of Cu.
Free Cu must be chelated and delivered to its physiological partner proteins by Cu chaperones after Cu uptake from the environment.Citation1,Citation2 Arabidopsis has at least three identified Cu chaperones, including the Cu chaperone for superoxide dismutase (SOD; CCS) and two homologs of yeast Antioxidant Protein1 (ATX1), Copper Chaperone (CCH) and ATX1.Citation3-Citation5 Results from our previous study suggested that ATX1 and CCH have different homeostatic properties and distinct functions in planta.Citation6 ATX1 in Arabidopsis contributes to tolerance of both excess and deficient Cu.Citation6 In previous study, we also found high Cu accumulation and tolerance in ATX1 overexpression lines grown in high-Cu–containing soil. These physiological functions of ATX1 depend on its Cu-binding motif MXCXXC.Citation6 ATX1 possesses excellent Cu-chelating activity and must be strictly regulated.Citation7
Here, we found that overexpression of ATX1 resulted in hypersensitivity to severe Cu deficiency. By contrast, overexpression of mutated ATX1 did not show the hypersensitivity. Regulation of ATX1 may have an important role in Cu homeostasis contributing to plant growth and development.
Cu is indispensable for all growth stages of higher plants.Citation8 Cu deficiency has been found throughout the world in all climatic zones where crops are grown or animals are kept on farms.Citation3,Citation9 Slight Cu deficiencies occur in many crops and cause up to 20% loss in yield without obvious symptoms.Citation9 Therefore, optimizing Cu availability in plants to maintain plant growth under Cu deficiency is important.
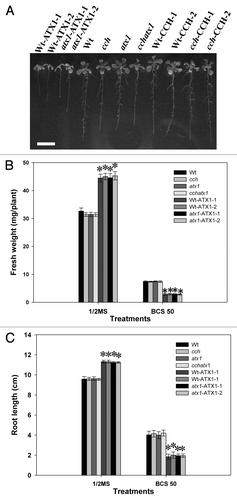
In a previous study, we demonstrated that overexpression of ATX1 enhanced tolerance to slight Cu deficiency in Arabidopsis. To investigate whether the overexpression of ATX1, with its excellent chelating activity, has any negative effects under Cu deficiency, Arabidopsis transgenic plants overexpressing ATX1 in a wild-type and atx1 mutant background (Wt-ATX1 and atx1-ATX1, respectively) were challenged with severe Cu deficiency. Wt-ATX1–1, Wt-ATX1–2, atx1-ATX1–1 and atx1-ATX1–2 were hypersensitive to severe Cu deficiency (). Fresh weight and root length were lower for Wt-ATX1–1, 2 and atx1-ATX1–1 than the wild type and the cch, atx1 and cchatx1 mutants under severe Cu deficiency (). The fresh weight for Wt-ATX1–1, Wt-ATX1–2, atx1-ATX1–1 and atx1-ATX1–2 was 40%, 39%, 48% and 42%, respectively, that of the wild type. In addition, the root length was about 46%, 50%, 48% and 48%, respectively, that of the wild type. However, the response of CCH transgenic lines and the wild type was similar ().
Figure 1. Growth of the wild type (Wt), Cu chaperone mutants (cch, atx1 and cchatx1) and transgenic plants with overexpression of Arabidopsis Antioxidant Protein1 (ATX1) (Wt-ATX1–1, Wt-ATX1–2, atx1-ATX1–1 and atx1-ATX1–2) or Copper Chaperone (CCH) (Wt-CCH-1, Wt-CCH-2, cch-CCH-1 and cch-CCH-2) under severe Cu deficiency. A, Seeds of plants were grown vertically on half-strength MS agar plates with 50 μM Cu chelator bathocuproine disulfonate (BCS) for 17 d (A). Bar = 1 cm. Plants were grown in half-strength MS medium and treated with BCS 50 μM for 17 d, and fresh weight (B) and root length (C) were measured. Data are mean ± SD of 4 replicates with 40 seedlings each. Student’s t-test was used for statistical analysis. * p < 0.01 compared with the wild type under the same condition. The plant materials and growth conditions were described previously.Citation6
To elucidate whether the MXCXXC Cu-binding motif of ATX1 plays a role in the hypersensitivity, we examined Arabidopsis transgenic plants overexpressing ATX1 with MXCXXC Cu-binding motif replaced by MXGXXG in a wild-type and atx1 mutant background (Wt-CG and atx1-CG, respectively) under severe Cu deficiency.Citation6 Wt-CG-1, Wt-CG-2, atx1-CG-1 and atx1-CG-2 transgenic lines were as sensitive as the wild type to severe Cu deficiency, and the biomass and root length were similar for Wt-CG-1, Wt-CG-2, atx1-CG-1, atx1-CG-2 and the wild type (data not shown). Therefore, ATX1-mediated hypersensitivity to severe Cu deficiency depends on the MXCXXC Cu-binding motif, and Cu chelation of ATX1 is crucial in the hypersensitivity to severe Cu deficiency. Overexpression ATX1 may create a critical cellular situation with exhausted Cu under severe Cu deficiency that retards plant growth.
Together with the previous report,Citation6 our study suggests that optimal levels of ATX1 protein ensure important functions in the control of Cu availability to affect plant growth and development.
Acknowlegments
This work was supported by the National Science Council (NSC 97–2311-B-001–008-MY3) and Academia Sinica.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Puig S, Andrés-Colás N, García-Molina A, Peñarrubia L. Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ 2007; 30:271 - 90; http://dx.doi.org/10.1111/j.1365-3040.2007.01642.x; PMID: 17263774
- Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M. Copper homeostasis. New Phytol 2009; 182:799 - 816; http://dx.doi.org/10.1111/j.1469-8137.2009.02846.x; PMID: 19402880
- Himelblau E, Mira H, Lin SJ, Culotta VC, Peñarrubia L, Amasino RM. Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol 1998; 117:1227 - 34; http://dx.doi.org/10.1104/pp.117.4.1227; PMID: 9701579
- Chu CC, Lee WC, Guo WY, Pan SM, Chen LJ, Li HM, et al. A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol 2005; 139:425 - 36; http://dx.doi.org/10.1104/pp.105.065284; PMID: 16126858
- Puig S, Mira H, Dorcey E, Sancenón V, Andrés-Colás N, Garcia-Molina A, et al. Higher plants possess two different types of ATX1-like copper chaperones. Biochem Biophys Res Commun 2007; 354:385 - 90; http://dx.doi.org/10.1016/j.bbrc.2006.12.215; PMID: 17223078
- Shin LJ, Lo JC, Yeh KC. Copper chaperone antioxidant protein 1 is essential for copper homeostasis. Plant Physiol 2012; In press http://dx.doi.org/10.1104/pp.112.195974; PMID: 22555879
- Shoshan MS, Tshuva EY. The MXCXXC class of metallochaperone proteins: model studies. Chem Soc Rev 2011; 40:5282 - 92; http://dx.doi.org/10.1039/c1cs15086c; PMID: 21695339
- Marschner H. Mineral Nutrition of Higher Plants. London: Academic Press, 1995.
- Graham RD, Nambiar EKS. Advances in Research on Copper Deficiency in Cereals. Aust J Agric Res 1981; 32:1009 - 37