Abstract
Endoreduplication is a cell cycle variant in which multiple rounds of DNA replication occur without subsequent mitosis, resulting in polyploid cells. Although cells with endoreduplicated nuclei were ubiquitously distributed throughout the abscission zone (AZ) of tomato leaf before abscission induction by ethylene, endoreduplication was detected mostly on the proximal side of the AZ after induction. The possible association between endoreduplication and intensive membrane trafficking in cells at the proximal side of the AZ is discussed.
Various organs are separated from the mother plant in a highly temporally and spatially regulated process of abscission.Citation1,Citation2 Abscission occurs in the pre-formed abscission zone (AZ) tissue located at the base of the organ to be shed. Although physiology and biochemistry of plant organ abscission have been studied intensively, the molecular mechanisms of these processes are still largely unknown. In tomato (Solanum lycopersicum) the plant hormones ethylene and auxin play central roles in regulating organ abscission by inducing and inhibiting it, respectively. Deblading of leaves or removal of flowers accelerates the abscission process by reducing the auxin supply,Citation3-Citation5 but the process may be even faster when the plants are additionally exposed to ethylene. We have recently shown that AZs of tomato leaf or flower are structured in an asymmetric manner after abscission is triggered ().Citation5 Thus, cells comprising the proximal side of the AZ, which is retained on the mother plant after the execution of abscission, differ substantially from those in the shed distal side in their morphology, biochemistry and metabolism. While the main process occurring in cells at the distal side is programmed cell death, cells at the proximal side exhibit several features presumably related to extensive membrane trafficking and /or high metabolic activity. It has been proposed that cell types undergoing differentiation, putatively related to specific metabolic properties, may exit the classical cell cycle and enter the onset of endoreduplication whereby cells undergo successive rounds of genome duplication without going through mitosis (For a review see refs. Citation6–Citation8). As a consequence, endoreduplication leads to increase of nuclear DNA content and to endopolyploid cells. Several lines of evidence imply that ethylene may exert an effect on endoreduplication (For a review see ref. Citation8). To investigate whether endoreduplication is also involved in development and function of the AZs of tomato leaves, a detailed image cytometry analysis for measuring the amount of nuclear was performed.Citation9-Citation11
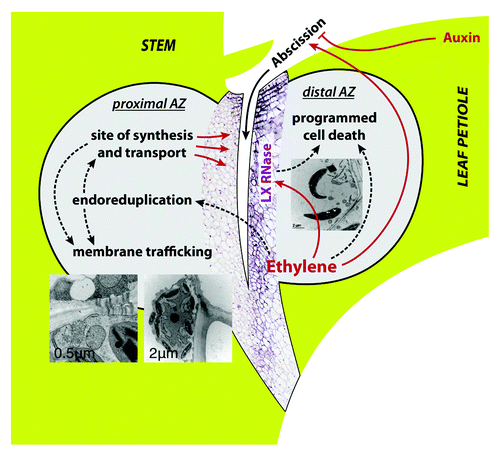
Figure 1. Model of the processes occurring in the AZ. A major process on the proximal side of the AZ is endoreduplication, presumably associated with extensive membrane trafficking. Its ultrastructure is characterized by electron-lucent ameboidal-shaped nuclei, a well developed Golgi apparatus and endoplasmic reticulum connected with an elaborate plasmodesmal system, pronounced membrane invaginations, and multivesicular and paramural bodies. In addition, polygalacturonases - enzymes involved in the execution of cell separationCitation4,Citation18 are also located at the proximal side of the AZ. At the distal side of the AZ programmed cell death occurs. It is identified by loss of cell viability, altered nuclear morphology, DNA fragmentation, elevated levels of reactive oxygen species, and elevated enzymatic activities and expression of PCD-associated genes, including genes encoding LX ribonuclease, nuclease TBN1, cysteine and serine proteases, tomato β-1,4-endoglucanase, which is involved in cell-wall metabolism; and 1-aminocyclopropane-1-carboxylic acid synthase, involved in ethylene biosynthesis.Citation5 Arrow-ended and blunt-ended lines represent process induction and repression, respectively, dashed lines represent putative relations.
Endoreduplication is Common in Stem and Leaf Tissues
Our study revealed a specific endoreduplication pattern in stem and leaf petiole tissues (). Adventitious buds as meristematic tissues consisted of diploid cells with a nuclear DNA content of 2 C and 4 C. Two C and 4 C nuclei were also localized around the petiole vascular tissue (). In contrast, all other stem and petiole tissues have endoreduplicated cells, regardless of the treatment. While 8 C nuclei were the most abundant, those with C-values 4 and 16 were frequent. In addition, some scattered 32 C nuclei were also present. Similar endoreduplication patterns have been discovered in tomato leaf petiole and stem by flow cytometry.Citation12
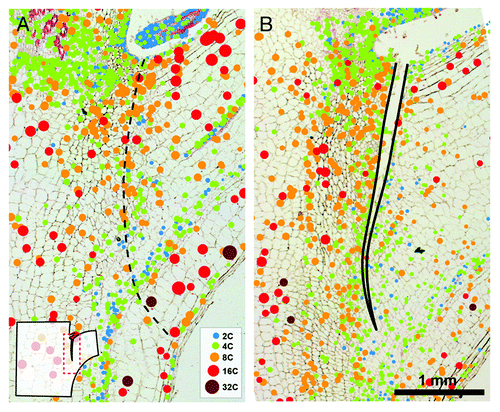
Figure 2. Endoreduplication in the AZ of untreated control (A) and debladed plant, 36 h after ethylene treatment (B). In situ DNA content of nuclei is shown as bubble graphs, superimposed over the images of Feulgen-stained median longitudinal tissue sections of AZ that were measured. For measuring the amount of nuclear DNA the interphase-peak method was applied.Citation9-Citation11,Citation19 The method adapted for use with tissue sections allows spatial and temporal examination of the distribution of nuclei. Nuclei of different endopolyploidy classes are color-coded in bubble graphs. Diameter of bubbles is linearly related to the diameter of measured nuclei. The site of future fracture is shown by dashed line in (A); fracture is delineated by black line in (B). The area visible in each panel is represented in the schematic drawing of a stem segment containing AZ in the lower left.
AZ is Endopolyploid Tissue
To compare the extent of endoreduplication in the AZ at different times after the induction of absiccision, we measured the tissue cycle value indicating the mean number of endoreduplication cycles per nucleus.Citation13 The cycle value of cells in the AZ of untreated control sample was between 1.08 and 1.30 () suggesting that AZ is endopolyploid tissue. Cycle values below 0.1 are considered to be non endopolyploid.Citation13 While most nuclei had C-values 4 C or 8 C (), some scattered 2 C and 16 C nuclei appeared in the control AZ.
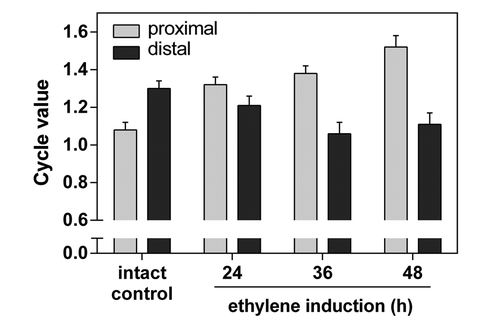
Figure 3. Cycle values of nuclei in proximal and distal side of AZs. The cycle value is calculated from the number of nuclei of each ploidy level multiplied by the number of endoreduplication cycles necessary to reach the corresponding ploidy level. The sum of the resulting products is divided by the total number of nuclei measured.Citation5 The cycle values were measured separately on proximal and distal side of AZs using nuclei distribution maps generated in ImageJ. Average cycle value and SD of five measurements per treatment are shown.
Endoreduplication in the AZ is Predominantly on its Proximal Side
Following the induction of abscission in young tomato plants by deblading of the four bottom leaves and ethylene treatment (For details see ref. Citation5), a progressive change in the distribution pattern of cells with endopolyploid nuclei was observed (). The cycle value () at the proximal side increased from 1.31 24 h after the ethylene treatment to 1.52 after 48 h. On the other hand, the cycle value decreased at the distal side from 1.24 24 h hours after ethylene treatment to 1.11 after 48 h. While on the proximal side cells with the endoreduplication level 8 C prevailed, at the distal side 4 C and 8 C nuclei were mixed with some 2 C ones ().
Deblading lowered the auxin concentration level in the AZ and made it sensitive to ethylene, which promoted the advancement of abscission.Citation14,Citation15 It has been shown that auxin has the opposite effect on endoreduplication as does ethylene, and mutants in auxin signaling, biosynthesis and transport show increased ploidy levels (For a review see ref. Citation8). In the presence of 1-aminocyclopropane-1-carboxylic acid, a direct precursor for ethylene biosynthesis, an extra round of endoreduplication was induced in Arabidopsis hypocotyl.Citation16 Similarly, during 24h and 48h-exposure of the epidermis of Cucumis sativus hypocotyls to ethylene, cells with 4 C to 8 C and up to 16 C DNA content were observed, respectively.Citation17 We have shown previously that 1-aminocyclopropane-1-carboxylic acid synthase gene (ACS) expression increased in the AZ.Citation5 Currently it is not known if the double effect of auxin and ethylene is involved in the induction of endoreduplication and the increased cycle value at the proximal side of the AZ.
It has been suggested that during plant development, endoreduplication occurs in metabolically active or highly specialized cells that could accommodate the enhanced transcription of nuclear genes needed for the assembly of organelles, leading to an increase in the numbers of organelles per cell (For a review see refs. Citation6). In addition, we have recently proposed that membrane trafficking through the multivesicular (MVB) and paramural bodies (PB) in cells of the proximal side of tomato AZ presumably participates in development of a protective layer at the surface of the exposed stem tissue by delivering and rapidly depositing endocytosed cell wall material at the cell surface following the release of cells at the site of leaf detachment from the plant body.Citation5 Accordingly, the observations of this study indicate that the increased endoreduplication level in cells at the proximal side of the AZ may support the observed increased amount of Golgi apparatus, endoplasmic reticulum connected to the extensive plasmodesmal system, ameboidal nuclei, MVBs and PBs ().Citation5
The fate of endopolyploid cells observed on the distal side of the AZ in the control but not after ethylene treatment is unknown at the moment. However, the association of their disappearance with the process of programmed cell death in the areaCitation5 may be considered based on circumstantial evidence suggesting that endoreduplication functions as a marker for cell death.Citation7,Citation20 On the other hand, plant cells, unlike animal cells, cannot migrate within tissues. Consequently, cell death usually leaves behind an opening in the local tissue structure. It has been recently suggested that Arabidopsis may acquire the strategy of actively inducing endoreduplication to prevent such gaps from arising in damaged tissue and thus to guarantee uninterrupted development during the life cycle.Citation21 It is possible therefore that PCD at the distal side of the AZ is a signal that induces endoreduplication and associated processes at the proximal side of the AZ. Understanding the molecular mechanisms of these phenomena will be the goal of future studies.
Acknowledgments
This research was supported by Research Grant J4–9738 from the Slovenian Research Agency, Research Grant 1019/06 from the Israel Science Foundation of the Israel Academy of Sciences; and Research Grants IS-3645–04. Authors are grateful to Dr. Elizabeth Covington for critical reading of the manuscript.
References
- Roberts JA, Elliott KA, González-Carranza ZH. Abscission, dehiscence, and other cell separation processes. Annu Rev Plant Biol 2002; 53:131 - 58; http://dx.doi.org/10.1146/annurev.arplant.53.092701.180236; PMID: 12221970
- Leslie ME, Lewis MW, Liljegren SJ. Organ abscission. In: Roberts JA and González-Carranza ZH, eds. Plant cell separation and adhesion. Oxford: Blackwell Publishing, 2007: 106-36.
- Meir S, Hunter DA, Chen JC, Halaly V, Reid MS. Molecular changes occurring during acquisition of abscission competence following auxin depletion in Mirabilis jalapa.. Plant Physiol 2006; 141:1604 - 16; http://dx.doi.org/10.1104/pp.106.079277; PMID: 16778017
- Meir S, Philosoph-Hadas S, Sundaresan S, Selvaraj KS, Burd S, Ophir R, et al. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol 2010; 154:1929 - 56; http://dx.doi.org/10.1104/pp.110.160697; PMID: 20947671
- Bar-Dror T, Dermastia M, Kladnik A, Znidaric MT, Novak MP, Meir S, et al. Programmed cell death occurs asymmetrically during abscission in tomato. Plant Cell 2011; 23:4146 - 63; http://dx.doi.org/10.1105/tpc.111.092494; PMID: 22128123
- Joubès J, Chevalier C. Endoreduplication in higher plants. Plant Mol Biol 2000; 43:735 - 45; http://dx.doi.org/10.1023/A:1006446417196; PMID: 11089873
- Dermastia M. Endoreduplication in cereal plants. In: Devane, Robert T, ed. New plant physiology research. New York: Nova Science Publishers, 2009:177-202.
- De Veylder L, Larkin JC, Schnittger A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci 2011; 16:624 - 34; http://dx.doi.org/10.1016/j.tplants.2011.07.001; PMID: 21889902
- Vilhar B, Greilhuber J, Dolenc Koce J, Temsch E, Dermastia M. Plant genome size measurement with DNA image cytometry. Ann Bot (Lond) 2001; 87:719 - 28; http://dx.doi.org/10.1006/anbo.2001.1394
- Vilhar B, Dermastia M. Standardisation of instrumentation in plant DNA image cytometry. Acta Bot Croat 2002; 61:11 - 25
- Vilhar B, Kladnik A, Blejec A, Chourey PS, Dermastia M. Cytometrical evidence that the loss of seed weight in the miniature1 seed mutant of maize is associated with reduced mitotic activity in the developing endosperm. Plant Physiol 2002; 129:23 - 30; http://dx.doi.org/10.1104/pp.001826; PMID: 12011334
- Smulders MJM, Rus-Kortekaas W, Gilissen LJW. Development of polysomaty during differentiation in diploid and tetraploid tomato (Lycopersicon esculentum) plants. Plant Sci 1994; 97:53 - 60; http://dx.doi.org/10.1016/0168-9452(94)90107-4
- Barow M, Meister A. Endopolyploidy in seed plants is differently correlated to systematics, organ, life strategy and genome size. Plant Cell Environ 2003; 26:571 - 84; http://dx.doi.org/10.1046/j.1365-3040.2003.00988.x
- Abeles FB, Rubinstein B. Regulation of ethylene evolution and leaf abscission by auxin. Plant Physiol 1964; 39:963 - 9; http://dx.doi.org/10.1104/pp.39.6.963; PMID: 16656043
- Sexton R, Roberts JA. Cell biology of abscission. Annu Rev Plant Physiol Plant Mol Biol 1982; 33:133 - 62
- Gendreau E, Orbovic V, Höfte H, Traas J. Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyl. Planta 1999; 209:513 - 6; http://dx.doi.org/10.1007/PL00008123; PMID: 10550633
- Dan H, Imaseki H, Wasteneys GO, Kazama H. Ethylene stimulates endoreduplication but inhibits cytokinesis in cucumber hypocotyl epidermis. Plant Physiol 2003; 133:726 - 31; http://dx.doi.org/10.1104/pp.103.025783; PMID: 14526115
- Kalaitzis P, Solomos T, Tucker ML. Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern. Plant Physiol 1997; 113:1303 - 8; http://dx.doi.org/10.1104/pp.113.4.1303; PMID: 9112778
- Dermastia M, Kladnik A, Dolenc Koce J, Chourey PS. A cellular study of teosinte Zea mays subsp. parviglumis (Poaceae) caryopsis development showing several processes conserved in maize. Am J Bot 2009; 96:1798 - 807; http://dx.doi.org/10.3732/ajb.0900059; PMID: 21622300
- Sabelli PA. Replicate and die for your own good: Endoreduplication and cell death in the cereal endosperm. J Cereal Sci 2012; 56:9e20
- Adachi S, Minamisawa K, Okushima Y, Inagaki S, Yoshiyama K, Kondou Y, et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis.. Proc Natl Acad Sci U S A 2011; 108:10004 - 9; http://dx.doi.org/10.1073/pnas.1103584108; PMID: 21613568