Abstract
Most living organisms on the earth have the circadian clock to synchronize their biochemical processes and physiological activities with environmental changes to optimize their propagation and survival. CIRCADIAN CLOCK-ASSOCIATED1 (CCA1) is one of the core clock components in Arabidopsis. Notably, it is also associated with cold acclimation. However, it is largely unknown how CCA1 activity is modulated by low temperatures. We found that the CCA1 activity is self-regulated by a splice variant CCA1β and the CCA1β production is modulated by low temperatures, linking the circadian clock with cold acclimation. CCA1β competitively inhibits the activities of functional CCA1α and LATE ELONGATED HYPOCOTYL (LHY) transcription factors by forming nonfunctional CCA1α-CCA1β and LHY-CCA1β heterodimers. Consequently, CCA1β-overexpressing plants (35S:CCA1β) exhibit shortened circadian periods as observed in cca1 lhy double mutants. In addition, elongated hypocotyls and petioles and delayed flowering of CCA1α-overexpressing plants (35S:CCA1α) were rescued by coexpression of CCA1β. Interestingly, low temperatures suppress CCA1 alternative splicing and thus derepress the CCA1α activity in inducing cold tolerance. These observations indicate that a cold-responsive self-regulatory circuit of CCA1 plays a role in plant responses to low temperatures.
The earth’s rotation on its axis causes rhythmic environmental changes. Therefore, most living organisms possess the circadian clock to anticipate upcoming environmental changes and synchronize many biological processes, such as stress responses, hormone signaling, metabolism, growth, and development, with the surroundings.Citation1,Citation2
Light and temperature are two major determinants that affect the circadian clock. The effect of ambient temperature changes in the circadian clock has been examined in both animals and plants, such as Neurospora, Drosophila, and Arabidopsis.Citation1,Citation3-Citation6 An additional temperature-related characteristics of the clock is temperature compensation, which refers to the maintenance of circadian periods within the physiological range of changes in ambient temperatures.Citation1,Citation7,Citation8
It has been shown that circadian clock-defective mutants show alterations in freezing tolerance, further supporting the significance of the clock in plant responses to cold temperatures.Citation9-Citation14 GIGANTEA (GI), which constitutes the clock output pathway, is involved in a cold response pathway that is associated with sugar accumulation in a C-repeat (CRT)/dehydration responsive element-binding factor (CBF)-independent manner.Citation11,Citation12 Clock central oscillators also regulate the CBF-COLD-REGULATED (COR) regulon, a major cold response module. For example, prr7 prr9 prr5 triple mutants exhibit enhanced cold resistance with elevated CBF and COR expression and accumulation of raffinose and proline.Citation13 In addition, CCA1 and LHY transcription factors regulate the expression of CBF genes by binding directly to the gene promoters.Citation14 Together, these observations suggest that the clock is closely associated with cold response in plants.
The activities of transcription factors are regulated at various steps, such as transcriptional and posttranscriptional regulation, posttranslational modifications, dynamic protein-protein interactions, and protein turnover.Citation15 Dynamic dimer formation modulates many aspects of transcription factor activities, for instance, DNA-binding affinity, transcriptional regulation activity, or subcellular localization. Recently, it has been shown that a group of small proteins that possess protein domains required for dimer formation but lack DNA-binding domains, including splice variants of transcription factors lacking functional protein domains, form nonfunctional heterodimers with transcription factors to attenuate their activities.Citation16-Citation18
We recently found that CCA1 alternative splicing produces two isoforms, the full-size CCA1α and the truncated CCA1β. The CCA1β isoform has the protein-protein interaction domain that mediates dimer formation. However, it lacks the N-terminal MYB DNA-binding domain, unlike the CCA1α isoform.Citation19 CCA1β competitively inhibits CCA1α activity by forming nonfunctional heterodimers CCA1α-CCA1β and LHY-CCA1β, which have reduced DNA-binding affinities. The similar disruptions of circadian rhythmic patterns in 35S:CCA1β transgenic plants and cca1 lhy double mutants and the attenuation of 35S:CCA1α transgenic plants by CCA1β coexpression further support the dominant negative regulation of CCA1α by CCA1β.
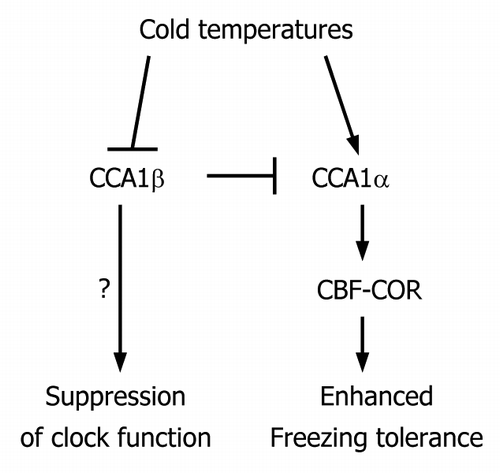
Of particular interest is the fact that alternative splicing of CCA1 is suppressed by low temperatures, resulting in upregulation of CCA1α activity.Citation19 Under cold conditions, CCA1α overcomes the repressible effects of CCA1β and turns out to be fully active in inducing freezing tolerance. Consistent with this, 35S:CCA1α transgenic plants exhibit enhanced freezing tolerance, whereas 35S:CCA1β transgenic plants are susceptible to freezing.Citation19 Thus, it is concluded that the CCA1α activity is modulated by CCA1β in response to low temperatures ().
Figure 1. Schematic model depicting the linkage between the clock and cold response. The CCA1 gene is alternatively spliced to produce two splice variants, CCA1α and CCA1β. Balanced CCA1 alternative splicing is physiologically important for the clock function under normal conditions. Under cold conditions, the CCA1 alternative splicing is suppressed, resulting in the reduction of CCA1β. As a result, the clock is suppressed, although underlying molecular mechanisms are not fully understood, and freezing tolerance is enhanced because of the elevation of CCA1α activity that induces expression of CBF genes. Therefore, the self-regulatory circuit of CCA1 underlies the signaling linkage between the clock function and cold response, which contributes to plant survival at low temperatures.
Recently, it has been reported that alternative splicing modulates the role of LHY in cold responses by producing nonfunctional transcripts.Citation20 Moreover, other clock components, such as TIMING OF CAB EXPRESSION1 (TOC1), PSEUDO RESPONSE REGULATOR3 (PRR3), PRR5, PRR7 and PRR9, are also alternatively spliced.Citation20 Circadian rhythms were disrupted in plants having mutations in PROTEIN ARGININE METHYLTRANSFERASE 5 (PRMT5) that catalyzes arginine methylation in the splicing factor responsible for PRR5 alternative splicing.Citation20 In Drosophila, a PRMT5 homolog also regulates alternative splicing of clock genes.Citation22 In addition, in Neurospora, alternative splicing of FREQUENCY (FRQ) mediates clock control of ambient temperature responses.Citation23 It therefore appears that alternative splicing of circadian clock genes is a widespread molecular mechanism that mediates clock function in eukaryotes.
Alternative splicing of pre-mRNAs occurs in splicesome consisting of five small nuclear ribonuleoproteins (snRNPs) and many non-snRNP proteins.Citation24 In Arabidopsis, approximately 70 snRNP genes and 400 genes encoding splicesomal and splicesome-associated proteins have been identified.Citation25 Recent reports indicate that genes encoding splicing regulators, such as serine/arginine-rich (SR) proteins, are also alternatively spliced in response to abiotic stresses, including cold and heat, which in turn alter the splicing of other pre-mRNAs.Citation24 Considering the alternative splicing of clock components and its relevance in regulating clock function in a temperature-dependent manner, studies on splicing factors or splicesomes will give more insights into understanding the link between the circadian clock and temperature responses.
Acknowledgments
This work was supported by the Leaping Research Program (20110016440) provided by the National Research Foundation of Korea, the Next-Generation BioGreen 21 program (Plant Molecular Breeding Center No. PJ008103) provided by the Rural Development Administration, and by a grant from the Agricultural R and D Promotion Center (309017–03), Korea Ministry for Food, Agriculture, Forestry and Fisheries.
References
- McClung CR. The genetics of plant clocks. Adv Genet 2011; 74:105 - 39; http://dx.doi.org/10.1016/B978-0-12-387690-4.00004-0; PMID: 21924976
- de Montaigu A, Tóth R, Coupland G. Plant development goes like clockwork. Trends Genet 2010; 26:296 - 306; http://dx.doi.org/10.1016/j.tig.2010.04.003; PMID: 20483501
- Rensing L, Ruoff P. Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol Int 2002; 19:807 - 64; http://dx.doi.org/10.1081/CBI-120014569; PMID: 12405549
- Pregueiro AM, Price-Lloyd N, Bell-Pedersen D, Heintzen C, Loros JJ, Dunlap JC. Assignment of an essential role for the Neurospora frequency gene in circadian entrainment to temperature cycles. Proc Natl Acad Sci U S A 2005; 102:2210 - 5; http://dx.doi.org/10.1073/pnas.0406506102; PMID: 15677317
- Glaser FT, Stanewsky R. Synchronization of the Drosophila circadian clock by temperature cycles. Cold Spring Harb Symp Quant Biol 2007; 72:233 - 42; http://dx.doi.org/10.1101/sqb.2007.72.046; PMID: 18419280
- Michael TP, McClung CR. Phase-specific circadian clock regulatory elements in Arabidopsis. Plant Physiol 2002; 130:627 - 38; http://dx.doi.org/10.1104/pp.004929; PMID: 12376630
- Gould PD, Locke JC, Larue C, Southern MM, Davis SJ, Hanano S, et al. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell 2006; 18:1177 - 87; http://dx.doi.org/10.1105/tpc.105.039990; PMID: 16617099
- Salomé PA, Weigel D, McClung CR. The role of the Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. Plant Cell 2010; 22:3650 - 61; http://dx.doi.org/10.1105/tpc.110.079087; PMID: 21098730
- Espinoza C, Bieniawska Z, Hincha DK, Hannah MA. Interactions between the circadian clock and cold-response in Arabidopsis. Plant Signal Behav 2008; 3:593 - 4; http://dx.doi.org/10.4161/psb.3.8.6340; PMID: 19704808
- Espinoza C, Degenkolbe T, Caldana C, Zuther E, Leisse A, Willmitzer L, et al. Interaction with diurnal and circadian regulation results in dynamic metabolic and transcriptional changes during cold acclimation in Arabidopsis. PLoS One 2010; 5:e14101; http://dx.doi.org/10.1371/journal.pone.0014101; PMID: 21124901
- Cao S, Ye M, Jiang S. Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis.. Plant Cell Rep 2005; 24:683 - 90; http://dx.doi.org/10.1007/s00299-005-0061-x; PMID: 16231185
- Cao S, Song Y, Su L. Freezing sensitivity in the gigantea mutant of Arabidopsis is associated with sugar deficiency. Biol Plant 2007; 51:359 - 62; http://dx.doi.org/10.1007/s10535-007-0073-1
- Nakamichi N, Kusano M, Fukushima A, Kita M, Ito S, Yamashino T, et al. Transcript profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR arrhythmic triple mutant reveals a role for the circadian clock in cold stress response. Plant Cell Physiol 2009; 50:447 - 62; http://dx.doi.org/10.1093/pcp/pcp004; PMID: 19131357
- Dong MA, Farré EM, Thomashow MF. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc Natl Acad Sci U S A 2011; 108:7241 - 6; http://dx.doi.org/10.1073/pnas.1103741108; PMID: 21471455
- Seo PJ, Hong SY, Kim SG, Park CM. Competitive inhibition of transcription factors by small interfering peptides. Trends Plant Sci 2011; 16:541 - 9; http://dx.doi.org/10.1016/j.tplants.2011.06.001; PMID: 21723179
- Hong SY, Kim OK, Kim SG, Yang MS, Park CM. Nuclear import and DNA binding of the ZHD5 transcription factor is modulated by a competitive peptide inhibitor in Arabidopsis. J Biol Chem 2011; 286:1659 - 68; http://dx.doi.org/10.1074/jbc.M110.167692; PMID: 21059647
- Seo PJ, Kim MJ, Ryu JY, Jeong EY, Park CM. Two splice variants of the IDD14 transcription factor competitively form nonfunctional heterodimers which may regulate starch metabolism. Nat Commun 2011; 2:303; http://dx.doi.org/10.1038/ncomms1303; PMID: 21556057
- Seo PJ, Hong SY, Ryu JY, Jeong EY, Kim SG, Baldwin IT, et al. Targeted inactivation of transcription factors by overexpression of their truncated forms in plants. Plant J 2012; •••; http://dx.doi.org/10.1111/j.1365-313X.2012.05069.x; PMID: 22672153
- Seo PJ, Park MJ, Lim MH, Kim SG, Lee M, Baldwin IT, et al. A self-regulatory circuit of CIRCADIAN CLOCK-ASSOCIATED1 underlies the circadian clock regulation of temperature responses in Arabidopsis. Plant Cell 2012; •••; http://dx.doi.org/10.1105/tpc.112.098723; PMID: 22715042
- James AB, Syed NH, Bordage S, Marshall J, Nimmo GA, Jenkins GI. Alternative splicing mediates responses of the Arabidopsis circadian clock to temperature changes.. Plant Cell 2012; 24:961 - 81; http://dx.doi.org/10.1105/tpc.111.093948; PMID: 22408072
- Hong S, Song HR, Lutz K, Kerstetter RA, Michael TP, McClung CR. Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana.. Proc Natl Acad Sci U S A 2010; 107:21211 - 6; http://dx.doi.org/10.1073/pnas.1011987107; PMID: 21097700
- Sanchez SE, Petrillo E, Beckwith EJ, Zhang X, Rugnone ML, Hernando CE, et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature 2010; 468:112 - 6; http://dx.doi.org/10.1038/nature09470; PMID: 20962777
- Liu Y, Garceau NY, Loros JJ, Dunlap JC. Thermally regulated translational control of FRQ mediates aspects of temperature responses in the neurospora circadian clock. Cell 1997; 89:477 - 86; http://dx.doi.org/10.1016/S0092-8674(00)80228-7; PMID: 9150147
- Reddy AS. Alternative splicing of pre-messenger RNAs in plants in the genomic era. Annu Rev Plant Biol 2007; 58:267 - 94; http://dx.doi.org/10.1146/annurev.arplant.58.032806.103754; PMID: 17222076
- Wang BB, Brendel V. The ASRG database: identification and survey of Arabidopsis thaliana genes involved in pre-mRNA splicing. Genome Biol 2004; 5:R102; http://dx.doi.org/10.1186/gb-2004-5-12-r102; PMID: 15575968