Abstract
The trans-Golgi network (TGN) contains multiple sorting domains and acts as the compartment for cargo sorting. Recent evidence indicates that the TGN also functions as an early endosome, the first compartment in the endocytic pathway in plants. The SYP4 group, plant Qa-SNAREs localized on the TGN, regulates both secretory and vacuolar transport pathways. Consistent with a secretory role, SYP4 proteins are required for extracellular resistance to fungal pathogens. However, the physiological role of SYP4 in abiotic stress remains unknown. Here, we report the phenotypes of a syp4-mutant in regard to salinity and osmotic response, and describe the physiological roles of the SYP4 group in the abiotic stress response.
Keywords: :
Membrane trafficking is one of the essential processes for plant growth, development, and the maintenance of cellular homeostasis. The SNARE family is one of the key regulators of membrane trafficking and executes appropriate membrane fusion between the transport vesicles and the membrane of the target organelle. SNARE proteins possess a highly conserved coiled-coil domain, called the SNARE domain, and are anchored to the membrane. On the basis of conserved amino acids (glutamine or arginine) in the “zero-layer” of the SNARE domain, the SNARE family has been classified as “Q-SNAREs” and “R-SNAREs.” The proteins are also classified into five classes: Qa-, Qb-, Qc-, R-SNAREs, and SNAPs. Q stands for glutamine and R for arginine - amino acid residues that are on the key position in the molecule. Q-SNAREs localize on the membrane of target organelles, whereas R-SNAREs localize on the transport vesicles.Citation1-Citation3
Recently, we reported the higher-order functions of the trans-Golgi network (TGN)-localized Qa-SNAREs, the SYP4 group consisting of SYP41, SYP42, and SYP43 in Arabidopsis.Citation4 Individual, single homozygous mutants (syp41, syp42, and syp43) were fully viable, but triple homozygous mutants (syp41−/− syp42−/− syp43−/−) were lethal due to impaired fertilization competence. The syp42−/− syp43−/− double mutant (syp42syp43) exhibited severe pleiotropic defects, including short roots, a large number of lateral roots, semi-dwarfism, and early senescence. Detailed analysis and observation of this double mutant revealed that SYP4 regulates the secretory and vacuolar transport pathways in the post-Golgi network and maintains the morphology of the Golgi apparatus and TGN. In addition, syp42syp43 was more susceptible to the non-adapted powdery mildew fungus Erysiphe pisi, whereas pathogenesis is terminated at an early stage with the wild-type. We noted a striking leaf chlorosis of syp42syp43 after pathogen challenge with conidiospores of the host-adapted virulent powdery fungus Golovinomyces orontii. This chlorosis was dependent on an intact pathogen-inducible salicylic acid biosynthesis pathway.Citation4 However, the physiological functions of SYP4 in abiotic stress have not yet been reported.
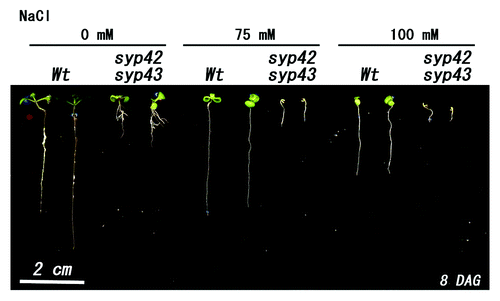
In our original paper, [4] syp42syp43 was mainly analyzed because this mutant is not lethal, though it does exhibit the dwarf and early senescence phenotypes. Since each single mutant for SYP41, SYP42, and SYP43 showed no abnormal phenotypes in abiotic stress response (data not shown), we analyzed syp42syp43 mutants in this study. We grew syp42syp43 in several types of media under abiotic stress conditions in order to reveal the physiological function of the SYP4 proteins in abiotic stress response. We found prominent phenotypes when syp42syp43 was grown in MS medium containing NaCl. The wild-type plant grown on MS medium with 75 mM NaCl exhibited slightly reduced growth compared with medium without NaCl, but the growth of syp42syp43 in MS medium with 75 mM NaCl was severely inhibited. In addition, syp42syp43 displayed greater sensitivity to salt when grown on MS medium supplemented with 100 mM NaCl. Moreover, syp42syp43 exhibited a nearly complete arrest of growth after cotyledon emergence (). These results clearly show that SYP4 proteins play critical roles in the salinity stress tolerance in plants.
Figure 1. Growth of syp42syp43 double mutants under salinity stress. Wild-type and the syp42syp43 double mutant were sown on MS medium containing 0, 75, or 150 mM NaCl and grown for 8 d at 22°C under continuous light. Growth of syp42syp43 was clearly inhibited. Scale bar = 2 cm.
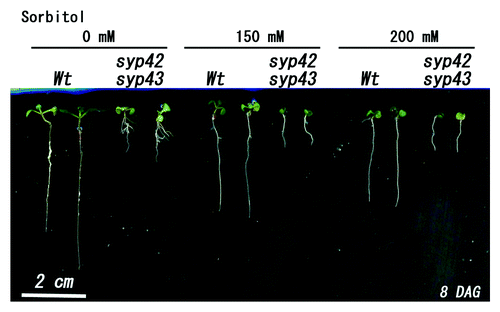
Next, the sensitivity of syp42syp43 to osmotic stress was investigated. When wild-type plants were grown on MS medium containing 150 or 200 mM sorbitol (hyper-osmotic medium), no clear growth differences were observed compared with MS medium without sorbitol. In contrast, syp42syp43 exhibited slightly reduced growth in hyper-osmotic medium (). These results imply a physiological role of SYP4 in the osmotic stress response in plants.
Figure 2. Growth of syp42syp43 double mutants under osmotic stress. Wild-type and the syp42syp43 double mutant were sown on MS medium containing 0, 150, or 200 mM sorbitol and grown for 8 d at 22°C under continuous light. Growth of syp42syp43 was reduced. Scale bar = 2 cm.
We have demonstrated that the SYP4 group plays important roles in both the biotic and abiotic stress response. As the SYP4 groupregulates the secretory and vacuolar transport pathways, describing the correlation between each physiological role and transport pathway regulated by SYP4 is difficult. The SYP4 proteins have been reported to form a SNARE complex with VTI12 (Qb-SNARE), SYP61 (Qc-SNARE), and YKT6 (R-SNARE).Citation5,Citation6 These results suggest that SYP4, VTI12, SYP61, and YKT6 are the components of the SNARE complex at the TGN. However, which transport pathway is regulated by the SNARE complex consisting of SYP4-VTI12-SYP61-YKT6 at the TGN is unclear. For example, the membrane fusion between the TGN and LE/MVB-derived vesicles in the retrograde pathway might be mediated by this SNARE complex, or endocytosed vesicles might fuse with the TGN under the control of this SNARE complex. This SNARE complex might also mediate membrane fusion between the transport vesicles budding from the trans-Golgi cisterna and the TGN.
SYP61 has been reported to localize on the TGN and function in both salinity and osmotic stress tolerance,Citation7 implying that the SNARE complex at the TGN plays important roles in the abiotic stress response. However, vti12 (a mutant of VTI12) exhibits accelerated leaf senescence similar to autophagy mutants, but not salinity and osmotic stress mutants, suggesting that VTI12 functions in the autophagy pathway.Citation8 Thus, several types of SNARE complexes on the TGN might also regulate the physiological roles in plants, though the number of SNAREs localized on the TGN is limited.
In order to dissect the transport pathways regulated by the SYP4 group in each abiotic stress response, the cargo molecules transported by SYP4 regulating pathways must be identified. Recently, intracellular Na+/H+ antiporters 5 and 6 (NHX5 and NHX6) were reported to localize on the TGN and the nhx5nhx6 double knockout mutant was shown to have reduced growth and an increased sensitivity to salinity.Citation9 From these results, we hypothesize that the subcellular localization of NHX5 or NHX6 is maintained by the transport pathways regulated by the SYP4 group. On the other hand, no molecule is known as a cargo candidate in the osmotic response. Further detailed analysis will reveal the functional link between SNARE complexes at the TGN and higher-order functions, such as the abiotic stress response in plants.
| Abbreviations: | ||
| SNARE | = | soluble N-ethylmaleimide sensitive factor attachment protein receptor |
| TGN | = | trans-Golgi network |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol 2006; 7:631 - 43; http://dx.doi.org/10.1038/nrm2002; PMID: 16912714
- Uemura T, Ueda T, Ohniwa RL, Nakano A, Takeyasu K, Sato MH. Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct Funct 2004; 29:49 - 65; http://dx.doi.org/10.1247/csf.29.49; PMID: 15342965
- Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol 2008; 15:658 - 64; http://dx.doi.org/10.1038/nsmb.1451; PMID: 18618939
- Uemura T, Kim H, Saito C, Ebine K, Ueda T, Schulze-Lefert P, et al. Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc Natl Acad Sci U S A 2012; 109:1784 - 9; http://dx.doi.org/10.1073/pnas.1115146109; PMID: 22307646
- Chen Y, Shin YK, Bassham DC. YKT6 is a core constituent of membrane fusion machineries at the Arabidopsis trans-Golgi network. J Mol Biol 2005; 350:92 - 101; http://dx.doi.org/10.1016/j.jmb.2005.04.061; PMID: 15919093
- Sanderfoot AA, Pilgrim M, Adam L, Raikhel NV. Disruption of individual members of Arabidopsis syntaxin gene families indicates each has essential functions. Plant Cell 2001; 13:659 - 66; PMID: 11251103
- Zhu J, Gong Z, Zhang C, Song CP, Damsz B, Inan G, et al. OSM1/SYP61: a syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 2002; 14:3009 - 28; http://dx.doi.org/10.1105/tpc.006981; PMID: 12468724
- Surpin M, Zheng H, Morita MT, Saito C, Avila E, Blakeslee JJ, et al. The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 2003; 15:2885 - 99; http://dx.doi.org/10.1105/tpc.016121; PMID: 14615598
- Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, et al. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 2011; 23:224 - 39; http://dx.doi.org/10.1105/tpc.110.079426; PMID: 21278129