Abstract
Several hormones have been studied for their effect on tuber initiation and development. Until recently, the hormone with the most prominent role in tuber initiation was attributed to GA. Genes involved in GA degradation do exhibit an upregulated profile during early stages of tuber development, leading to a rapid decrease of active GA content, thereby facilitating stolon-tip swelling. While GA is known to be involved in shoot and stolon elongation, the development of the new tuberorgan requires changes in meristem identity and the reorientation ofthe plane of cell division. In other developmental processes, such as embryo patterning, flower development and lateral root initiation auxin plays a key role. Recent evidence on the involvement of auxin in tuber formation was providedby the measurement of auxin content in swelling stolons. Auxin content in the stolon tips increased several fold prior to tuber swelling. In vitro tuberisation experiments with auxin applications support the role of auxin during tuber initiation. Taken together, it is becoming clear that the initiation and induction of tubers in potato is a developmental process that appears to be regulated by a crosstalk between GA and auxin.
Keywords: :
Several plant hormones have been studied for their effect on tuber initiation. The class of plant hormones that has been studied most extensively is the group of Gibberellic Acids (GAs). More than 120 GAs have been identified in plants, but only GA1 and GA4 are biologically active. GAs have an inhibitory role on tuber induction.Citation1-Citation3 Analysis of the endogenous GA levels show that after tuber induction, content of the active GAs in the swelling stolon tips is depleted,Citation4,Citation5 while application of active GAs inhibits initiation of tuberisation.Citation4,Citation6 Several GA biosynthesis genes have been identified in potato and their role in tuber initiation has been studied. The expression of StGA2ox1, which is involved in GA degradation, is induced prior to stolon swelling.Citation7 In agreement with these results, StGA20ox1 (active GAs biosynthesis) overexpression or antisense potato plants delayed or advanced the time point of tuberisation, respectively.Citation8 In addition, antisense transgenic plants for the StGA2ox1 gene had increased levels of GA20 that is an inactive form of GA, reduced stolon growth and earlier in vitro tuberisation. In contrast, overexpression of StGA2ox1 delays in vitro tuberisation and alters tuber morphology.Citation7 StGA3ox2 has been used in constructs with leaf, tuber specific or constitutive overexpression with the CaMV 35S promoter. The leaf specific expression of StGA3ox2 and the 35S overexpression resulted in earlier tuberisation, in contrast to the tuber specific expression that had slightly delayed tuberisation.Citation9 The different phenotypes observed when the StGA3ox2 gene expression is driven by the leaf or the tuber specific promoter can be explained by different transport of the various GAs. The main transporter in pea was shown to be GA20,Citation10 a precursor of GA1. Therefore, overexpression of StGA3ox2 in the leaves would convert GA20 to GA1 in the leaves more vigorously, resulting in lower GA20 being transported to the stolon tips. As a consequence, reduced GA20 availability in the stolon tips will result in reduced GA1 content that can lead to earlier tuberisation.
In addition to GAs, several other plant hormones have been studied for their effect on tuber initiation. Exogenous application of cytokinin (zeatinriboside) to an in vitro tuberisation system, resulted in increased tuber formation.Citation11 In addition, plants overexpressing a cytokinin biosynthesis gene (isopentenyltransferase gene- ipt) yielded more tubers with reduced tuber weight and nitrogen content,Citation12 suggesting that cytokinins may act more on promoting stolon branching than on tuber induction.Abscisic acid (ABA) applications on in vitro tuberisation systems have produced contradictary results. Koda and OkazawaCitation6 reported that application of ABA in an in vitro tuberisation system with 2% sucrose resulted in slight swellings in the sub apical region that did not develop into proper tubers. In contrast, Xu et al.Citation4 reported that ABA application resulted in higher frequencies of tubers only in 1% sucrose, but estimation of the ABA-like substances in tuberising explants were not different compared with non tuberising explants.
Other hormones such as jasmonic acid (JA) have also been implicated in the initiation of tuberisation. Application of JA in in vitro explants enhanced tuberisation.Citation13 In addition, an important increase in JA content was noticed at tuber set, but in tubers no changes in the content of JA was noticed.Citation14 Nevertheless, JA application experiments showed promotion or inhibition of tubersiation, depending on the concentrations applied.Citation2
Auxin plays a very important role in almost all developmental events in a plant that require changes in meristem identity (reviewed in reference Citation15).Cytological studies in the stolon tip revealed that upon tuber induction the apical meristem ceases cell divisionand the plane of cell division in the sub-apical medullary region changes from a lateral to a longitudinal thereby terminating stolon elongation and resulting in swelling.Citation16
Recently, Dhonukshe et al.Citation17 showed that the re-orientation of the plane of cell division of Arabidopsis stem cells is auxin dependent, demonstrating a possible auxin mediated mechanism that regulates changes in the orientation of cell division in plants. Therefore, changes in the orientation of cell division that results in swelling of the stolon tip are compatible with a role for auxin in tuber development.
Early experiments with applications of auxin in in vitro tuberisation systems did not provide a conclusive link between auxin and tuber development.Citation4,Citation5 The advent of the genomic era in biology provided new tools to study tuber initiation and development though the study of gene expression and function. The differential expression of an auxin response factor gene showing a peak in expression just after tuber initiation provided a first indication that auxin plays a role in tuber initiation.Citation18 A more comprehensive expression study using a microarray approach provided a much more detailed picture of the transcriptome wide regulation of genes during tuber initiation. This microarray experiment revealed that a large number of auxin related genes had a differential expression profile during early events in tuber development.Citation19 Examples of such genes are two PIN-like genes, an adr11–2 (auxin downregulated) and an acrA-like (auxin regulated gene containing a GTP-binding site) genes. In Arabidopsis, transcript levels of adr genes were shown to be downregulated in presence of auxin,Citation20 while in tobacco, acrAexpression levels are upregulated after auxin application.Citation21 In potato, transcript levels of the StPIN-like and the acrA-like gene exhibited a peak in expression after tuber initiation, while the adr-like gene was downregulated. These expression profiles indicate that auxin levels are likely to increase during early stages of tuber development. Scoring of auxin content in the stolon () reveal that after tuber initiation, auxin content indeed increases in the stolon tip and in the region just below the site of swelling where swelling occurs.
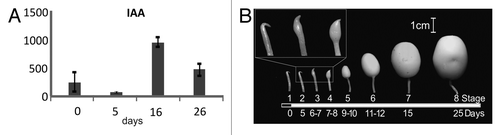
Figure 1. (A) Concentration of free IAA the stolon rip. Plants were grown for 9 weeks under non-inductive conditions, before switch to inductive short day conditions. IAA and OxIAA concentrations are in pmol per gram of fresh weight. Samples were harvested under long day conditions (LD day 0) just before switching to inductive short days and after 5, 8 and 26 d in short day conditions (SD day 5, 8 and 26 respectively). Error bars represent standard error of the mean of two replicated measurements. (B) Representation of stages of tuberisation before and after transition to inductive conditions (black section of the bar represents SD and white section represents LD). The days of growth are shown below. The inset in the top left, is a 3 × magnification of stages 2–4.
Furthermore, the analysis of auxin biosynthesis genes, as well as of PIN family of genes which is involved in auxin transport, it was verified that the at least one auxin biosynthesis gene, named StYUC-like1, is upregulated during early stages of tuber development. In addition, auxin transport assays revealed that IAA is directionally transported from the distal stolon apical meristem (STAM) to the proximal region of the stolon, strengthening the hypothesis that the stolon tip is a possible site of auxin biosynthesis.Citation22 Expression studies of the StPIN genes in potato in early stages if tuber initiation as introduced inCitation19 revealed that several genes exhibit an peak in expression profile after tuber induction (). Taken together, these results, combined with the known role for auxin in the development of other meristems, provides strong evidence for a role of auxin and auxin distribution in tuberisation and more specifically in the events that take place after tuber initiation.
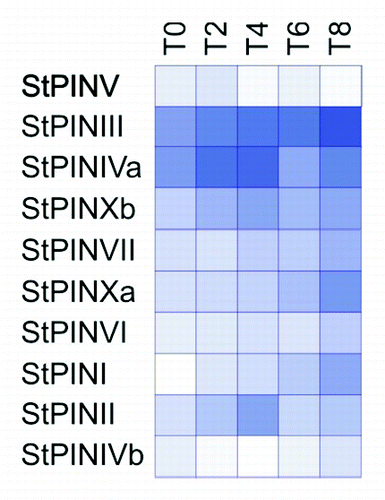
Figure 2. Heat map of expression of the StPINs in stages T0 to T8 of the developmental series, 0 to 8 d after induction to tuberise, shades of blue represent fold increase in the expression of the corresponding gene and white indicates the lowest expression detected. Lowest expression is detected for StPINVb at stage T4 (C(t) = 36.29), and highest expression is detected for StPINIII at stage T8 (C(t) = 25.14).
Our findings on auxin content together with the known role for GAs in tuber initiation and development, allows us to describe the physiological model for the combined action of GAs and auxin during early stages of tuberisation. GAs and auxin seem to be involved in tuber development in two consecutive stages (). At the stolon growth phase, GA content is relatively high and is mediating stolon elongation. The plain of cell division remains transversal during this phase. Auxin content is relatively low and the role of auxin is to maintain stolon apical dominance. Short day conditions induce tuber initiation, and the mobile signal StSP6A is produced and transported from the aerial parts of the plant to the stolon tips.Citation23 When the StSP6A protein reaches the stolon tip, tuber formation is induced. Concomitantly, GA levels are rapidly reduced and auxin content has a peak. This occurs together with termination of longitudinal stolon growth, change of the plane of cell division and swelling of the stolon to form the tuber. GA content is a very important switch in this developmental event. When GA degradation is hindered, a delay in the tuber initiation can occur, while greater GA degradation produces earlier tuberisation.Citation7
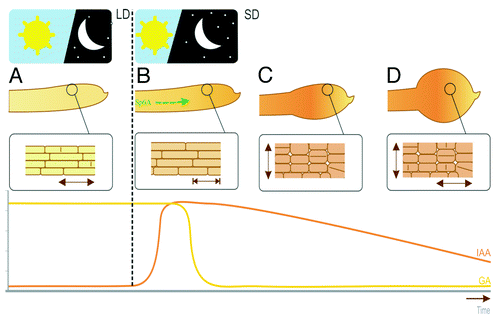
Figure 3. Changes in the GA and IAA content in the stolon tip. GA content is initially high and IAA content is low under non-inductive long day conditions. The stolon is elongating longitudinally (A). The black dotted line represents switch to tuber inductive long days. StSP6A transcript (green arrow) is the mobile signal that reaches the stolon tip. Elongation of the stolon is terminated (B), and GA content drops while IAA content is increased. Tuber swelling is achieved initially by cell transversal division (C) and finally by random cell divisions (D).
At the tuber swelling stage, GA content has been degraded, while IAA content remains high and slowly decreases over time, correlated with a peak in the expression of a StYUC gene. It is during this stage that changes in the orientation of cell division in the tuber take place to conduct tuber growth. In vitro experiments with auxin application also point out the importance of auxin in tuber initiation.Citation22 If auxin levels remain high by continuous application of auxin to the stolon tip, tuber formation is inhibited. On the other hand, a single auxin pulse in vitro stimulates tuberisation in comparison to the controls.Citation22 Therefore, the auxin peak that was found in vivo, is an important factor for a stolon to start swelling. The recent finding that the transcription factor KANADI interacts with FT in rice,Citation24 provides a possible mechanism for the dove-tailing of the light-regulation pathway with the auxin control of cell division in plants. Further research will show how this link may function in the potato system.
Based on these results, we conclude that auxin has an important role in tuber development, possibly by regulating tuber growth through mediating meristem identity, cell division and the plane of cell division.
Acknowledgments
E.R. gratefully acknowledges financial support from the Bakalas Foundation, and the StichtingVeenhuizen-Tulpfonds.
References
- Jackson SD, Prat S. Control of tuberisation in potato by gibberellins and phytochrome B. Physiol Plant 1996; 98:407 - 12; http://dx.doi.org/10.1034/j.1399-3054.1996.980224.x
- Vreugdenhil D, Struik PC. An integrated view of the hormonal regulation of tuber formation in potato (Solanum tuberosum). Physiol Plant 1989; 75:525 - 31; http://dx.doi.org/10.1111/j.1399-3054.1989.tb05619.x
- Ewing E. The role of hormones in potato (Solanum tuberosum L.) tuberization. Boston: Martinus Nijhoff Publishers, Boston, 1987.
- Xu X, Vermeer E, Vreugdenhil D, van Lammeren AA. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 1998; 117:575 - 84; http://dx.doi.org/10.1104/pp.117.2.575; PMID: 9625710
- Koda Y, Okazawa Y. Characteristic changes in the levels of endogenous plant hormones in relation to the onset of potato tuberization. Jpn J Crop Sci 1983; 52:592 - 7; http://dx.doi.org/10.1626/jcs.52.592
- Koda Y, Okazawa Y. Influences of invironmental, hormonal and nutritional factors on potato toberization in vitro. Jpn J Crop Sci 1983; 52:582 - 91; http://dx.doi.org/10.1626/jcs.52.582
- Kloosterman B, Navarro C, Bijsterbosch G, Lange T, Prat S, Visser RG, et al. StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J 2007; 52:362 - 73; http://dx.doi.org/10.1111/j.1365-313X.2007.03245.x; PMID: 17764503
- Carrera E, Bou J, García-Martínez JL, Prat S. Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J 2000; 22:247 - 56; http://dx.doi.org/10.1046/j.1365-313x.2000.00736.x; PMID: 10849342
- Bou-Torrent J, Martínez-García JF, García-Martínez JL, Prat S. Gibberellin A1 metabolism contributes to the control of photoperiod-mediated tuberization in potato. PLoS One 2011; 6:e24458; http://dx.doi.org/10.1371/journal.pone.0024458; PMID: 21961036
- Proebsting WM, Hedden P, Lewis MJ, Croker SJ, Proebsting LN. Gibberellin concentration and transport in genetic lines of pea : effects of grafting. Plant Physiol 1992; 100:1354 - 60; http://dx.doi.org/10.1104/pp.100.3.1354; PMID: 16653128
- Mauk CS, Langille AR. Physiology of tuberization in Solanum tuberosum L: cis-zeatin riboside in the potato plant: its identification and changes in endogenous levels as influenced by temperature and photoperiod. Plant Physiol 1978; 62:438 - 42; http://dx.doi.org/10.1104/pp.62.3.438; PMID: 16660533
- Tao G, et al. Promotion of shoot development and tuberisation in potato by expression of a chimaeric cytokinin synthesis gene at normal and elevated CO2 levels. Funct Plant Biol 2010; 37:43 - 54; http://dx.doi.org/10.1071/FP07032
- Pelacho AM, Mingo-Castel AM. Jasmonic Acid induces tuberization of potato stolons cultured in vitro. Plant Physiol 1991; 97:1253 - 5; http://dx.doi.org/10.1104/pp.97.3.1253; PMID: 16668517
- Abdala G, et al. Changes in jasmonate and gibberellin levels during development of potato plants (Solanum tuberosum). Plant Growth Regul 2002; 36:121 - 6; http://dx.doi.org/10.1023/A:1015065011536
- Möller B, Weijers D. Auxin control of embryo patterning. Cold Spring Harb Perspect Biol 2009; 1:a001545; http://dx.doi.org/10.1101/cshperspect.a001545; PMID: 20066117
- Xu X, Vreugdenhil D, van Lammeren AAM. Cell division and cell enlargement during potato tuber formation. J Exp Bot 1998; 49:573 - 82
- Dhonukshe P, Weits DA, Cruz-Ramirez A, Deinum EE, Tindemans SH, Kakar K, et al. A PLETHORA-auxin transcription module controls cell division plane rotation through MAP65 and CLASP. Cell 2012; 149:383 - 96; http://dx.doi.org/10.1016/j.cell.2012.02.051; PMID: 22500804
- Faivre-Rampant O, Cardle L, Marshall D, Viola R, Taylor MA. Changes in gene expression during meristem activation processes in Solanum tuberosum with a focus on the regulation of an auxin response factor gene. J Exp Bot 2004; 55:613 - 22; http://dx.doi.org/10.1093/jxb/erh075; PMID: 14966214
- Kloosterman B, Vorst O, Hall RD, Visser RG, Bachem CW. Tuber on a chip: differential gene expression during potato tuber development. Plant Biotechnol J 2005; 3:505 - 19; http://dx.doi.org/10.1111/j.1467-7652.2005.00141.x; PMID: 17173637
- Datta N, LaFayette PR, Kroner PA, Nagao RT, Key JL. Isolation and characterization of three families of auxin down-regulated cDNA clones. Plant Mol Biol 1993; 21:859 - 69; http://dx.doi.org/10.1007/BF00027117; PMID: 8096772
- Ishida S, Takahashi Y, Nagata T. Isolation of cDNA of an auxin-regulated gene encoding a G protein beta subunit-like protein from tobacco BY-2 cells. Proc Natl Acad Sci U S A 1993; 90:11152 - 6; http://dx.doi.org/10.1073/pnas.90.23.11152; PMID: 8248221
- Roumeliotis E, Kloosterman B, Oortwijn M, Kohlen W, Bouwmeester HJ, Visser RG, et al. The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J Exp Bot 2012; In press http://dx.doi.org/10.1093/jxb/ers132; PMID: 22689826
- Navarro C, Abelenda JA, Cruz-Oró E, Cuéllar CA, Tamaki S, Silva J, et al. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011; 478:119 - 22; http://dx.doi.org/10.1038/nature10431; PMID: 21947007
- Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 2011; 476:332 - 5; http://dx.doi.org/10.1038/nature10272; PMID: 21804566