Abstract
Jasmonates (JAs) are ubiquitously occurring signaling compounds in plants formed in response to biotic and abiotic stress as well as in development. (+)-7-iso-jasmonoyl isoleucine, the bioactive JA, is involved in most JA-dependent processes mediated by the F-box protein COI1 in a proteasome-dependent manner. However, there is an increasing number of examples, where the precursor of JA biosynthesis, cis-(+)-12-oxophytodienoic acid (OPDA) is active in a JA/COI1-independent manner. Here, we discuss those OPDA-dependent processes, thereby giving emphasis on tomato embryo development. Recent data on seed coat-generated OPDA and its role in embryo development is discussed based on biochemical and genetic evidences.
The plant hormone jasmonic acid (JA) was initially detected as its methyl ester in the essential oil of flowers of Jasminum grandiflorum.Citation1 In the sixties and the seventies numerous additional jasmonate compounds were detected preferentially in flowers.Citation2 In the last three decades hydroxylated, glucosylated, sulphated, decarboxylated and carboxylated derivatives as well as JA amino acid conjugates have been identified as plant constituents. Upon wounding there is a rapid accumulation of JA, JA-Ile within some minutes, followed by accumulation of their hydroxylated, carboxylated and glucosylated derivatives.Citation3-Citation5 Some of these JA metabolites occur in flower organs and seeds up to three orders of magnitude higher levels than JA.Citation6 All genes encoding enzymes in JA biosynthesis have been cloned from various plant species.Citation7,Citation8 Genes encoding enzymes of JA metabolism are partially cloned. Most recently, hydroxylation and carboxylation of the pentenyl side chain of JA and JA-Ile were characterized in terms of genes and enzymes involved.Citation9-Citation11 This metabolic conversion of JA and JA-Ile, respectively, leads to compounds being biologically inactive in some processes such as wounding, where JA and JA-Ile are typically involved as signaling compounds.Citation6,Citation9,Citation11 In contrast, an O-glycosylated JA derivative is active in leaf movement,Citation12 and decarboxylated JA, called cis-jasmone, leads to expression of a specific set of genes different from those involved in JA-induced gene expression.Citation13 All these aspects contribute to the questions whether or not the different JA compounds exhibit individual specificity in signaling and which of the metabolic conversion of JA or JA-Ile represents a “switch off” in its signaling cascade.
In the last couple of years a similar question was already addressed for the precursor of JA, 12-oxophytodienoic acid (OPDA). Cis-(+)-OPDA is formed by the allene oxide cyclase (AOC)-catalyzed step of JA biosynthesis () leading to establishment of that enantiomeric structure which is a feature of the naturally occurring JA.Citation7,Citation14 OPDA accumulates upon wounding of leaves usually to higher levels than JA and JA-Ile.Citation3,Citation4,Citation15 Moreover, a large portion of OPDA is esterified within lipid membranes to numerous compounds.Citation16,Citation17 These compounds are called arabidopsides due to their exclusive occurrence in the genus Arabidopsis. Interestingly, OPDA occur constitutively in different flower organs of tomato.Citation18 Several mutants, initially identified in Arabidopsis were also characterized for tomato. The spr2 mutant is affected in the generation of α-LeA due to a block in ω-7-fatty acid desaturase,Citation19 thus being the homolog of the triple mutant fad3-2fad7-2fad8 of Arabidopsis.Citation20 The tomato mutant acx1 is affected in the conversion of reduced OPDA into the oxo-pentenyl-cyclopentane-(OPC)-derivative shortened by two carbon atoms of the carboxylic acid side chain due to β-oxidation. Consequently, the spr2 mutant is deficient in α-LeA, OPDA and JA, whereas the acx1 mutant is deficient in JA, but does accumulate OPDA. In this respect the acx1 mutant is similar to the Arabidopsis mutant opr3 which is affect in the reduction of OPDA upstream of the ACX1-catalyzed stepCitation21 (). The most prominent mutant in JA signaling is coi1 initially identified in Arabidopsis. COI1 encodes an F-box protein being part of the JA-Ile co-receptor complex.Citation22 Its loss of function results in a male sterile phenotype of the coi1 mutant.Citation23 Mutations in the tomato homolog of COI1, called JAI1, affects the female gametophyte development: The corresponding mutant jai1 is female sterile.Citation24 This suggests that the same process in terms of JA perception can have a different function in different species.Citation2
Figure 1. Schematic overview of the biosynthesis of jasmonates and jasmonate signal transduction leading to specific gene expression. The main intermediates as well as the bioactive compound, (+)-7-iso-jasmonoyl isoleucine, are shown. Mutants characterized for tomato and Arabidopsis are indicated at the left and the right, respectively. Abbreviations of enzymes: LOX, lipoxygenase; AOS, allene oxide synthase; AOC, allene oxide cyclase; OPR3, OPDA reductase3; ACX, acyl-CoA oxidase, MFP, multifunctional protein, KAT, 3-ketoacyl-CoA-thiolase; JAR1, jasmonoyl-l-isoleucine synthase.
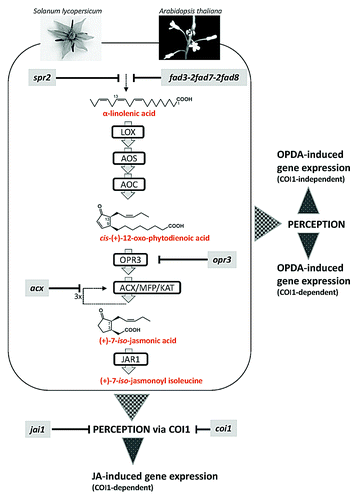
Recently, a jai1 like phenotype of flowers was observed in 35S::AOC-RNAi lines which have reduced level of AOC in ovules.Citation25 The AOC-catalyzed step in JA biosynthesis is required for OPDA and JA generation. Therefore, the flower phenotype of 35S::AOC-RNAi lines was assumed to be caused by any compound formed downstream of the AOC step. Due to the low seed set of these RNAi lines, however, OPDA and JA levels could not be analyzed.
Interestingly, the tomato mutant spr2 has a delay in proper embryo development. The acx1 mutant, however, showed in the curled cotyledon stage of embryo development a phenotype similar to the wild type. These data suggest that a compound generated downstream of the fatty acid desaturase and upstream of ACX1 is required for proper embryo development. One putative candidate was OPDA. Indeed, OPDA was the dominant compound in the seed coat of wild type seed and accumulated in the acx1 seeds.Citation25 Increased programmed cell death (PCD) monitored by the TUNEL assay was observed in the seed coat and endosperm of spr2 seeds compared with that of wild type seeds. To proof whether OPDA was the essential compound for embryo development, a rescue of the embryo phenotype of spr2 seed was tried by OPDA treatment. The permanent generation, however, of JA upon injection of OPDA into the fruits of the corresponding stage of embryo development did not allow a clear cut answer.
Similarly, a residual amount of JA and JA-Ile detected in the acx1 seeds could be sufficient for the normalized phenotype of acx1 embryos. Therefore, the jai1 mutant was used to distinguish clearly between JA and OPDA. JA perception requires interaction of the most bioactive JA compound (+)-7-iso-JA-IleCitation26 with COI1 and JAZ proteins, the repressors of JA induced gene expression.Citation27-Citation29 The interaction takes place in the COI1-JAZ co-receptor complex which has been crystallized.Citation22 Surprisingly, this complex does not bind OPDA,Citation28,Citation30 and OPDA does not fit into the binding pocket.Citation29 Consequently, OPDA activity could be recorded with the jai1 mutant without any overlay by JA perception. The above described embryo phenotype of jai1 flowers could be partially rescued by endogenously formed OPDA in repeatedly wounded plants compared with un-wounded plants.Citation25 All these data suggest a COI1-independent role of seed coat-generated OPDA in embryo development of tomato.
Although there is no mechanistic explanation so far, how OPDA is perceived, at least two scenarios for the described link between OPDA and tomato embryo development can be discussed:
(i) The high OPDA level in the seed coat may lead to expression of stress responsive genes such as those encoding glutathione-S-transferase or heat shock proteins and being known to be specifically upregulated by OPDA.Citation31,Citation32 Such expression of stress-responsive genes may influence nutritional supply during embryo development. This is supported by the fact that in the spr2 mutant, which is unable to form OPDA, cells of the seed coat undergoes increased PCD.
(ii) Finally, a link between carbon availability and seed coat-generated OPDA may occur: In tomato the cell wall invertase 5 (LIN5) specifically expressed in gynoeciaCitation33 is required for sufficient number of seeds per fruit and for proper fruit yield. LIN5 downregulated plants showed a decreased content of JA and hexoses.Citation34 Moreover, the LIN5 activity is regulated post-translational by the LIN5-specific inhibitor protein INVINH1. INVINH1 is co-expressed with LIN5,Citation35 but specifically downregulated by OPDA.Citation32 Obviously, the seed coat-generated OPDA shown for tomato embryo developmentCitation25 attribute to generation of hexoses via altered ratio of LIN5/INVINH1 activity.
Any OPDA-specific response leads to the question, how OPDA is perceived. There is now mechanistic explanation so far for OPDA perception due to the above mentioned fact that the COI1-JAZ-coreceptor complex does not bind OPDA. There is however, an increasing number of examples on OPDA-specific signaling:
(i) Initially, an OPDA-specific process was suggested by endogenous rise of OPDA but not JA during tendril coiling of Bryonia dioica.Citation36 In that plants a much more rapid coiling-response occurs upon treatment with OPDA than with JA.Citation37
(ii) Later on, genes were identified which are specifically expressed by OPDA.Citation31,Citation32 Among them are stress responsive genes.
(iii) A surprising example is given by the OPDA-induced expression of AtPHO1;H10, a member of the PHO1- gene family involved in numerous stress responses. AtPHO1;H10 is expressed COI1-dependently but distinct from JA.Citation38 This suggests the existence of additional signaling component(s) of COI1-mediated signaling which exhibits OPDA dependency but JA independency. A similar example was observed upon infection of Arabidopsis shoots with the vascular pathogen Verticillium longisporum.Citation39 Here, COI1 activity is required but is independent of JA/JA-Ile. Furthermore, the promoter of CYP81D11, a gene encoding a putative cytochrome P450 monooxygenase active in detoxification, functions COI1-dependently in the absence of JA/JA-Ile.Citation40
(iv) In the moos Physcomitrella patens which is unable to form JA but accumulate OPDA, KO- lines of the AOC are affected in fertility suggesting requirement of OPDA.Citation41
(v) OPDA was shown to be involved in phytochrome A signaling and shade avoidanceCitation42 as well as in hypocotyl growth inhibition at reduced V-ATPase activity.Citation43
(vi) Strong support on COI1-independent, OPDA-dependent signaling came recently from data on seed germination in Arabidopsis.Citation44 Here, OPDA but not JA is the inhibitory compound of seed germination inhibition, a process known for a long time.Citation44,Citation45 Genetic evidence revealed a synergistic action of the seed germination antagonist ABA.
(vii) Furthermore, OPDA specific functions are suggested by occurrence of cis-OPDA and dinor-OPDA in galactolipids esterified in the sn-1 and/or sn-2 position. These compounds —the arabidopsides—are rapidly formed and accumulate to high levels under different stress conditions.Citation16,Citation17,Citation45 After disruption of cellular integrity arabidopsides are formed from esterified fatty acids indicating that LOX, AOS and AOC act on membrane-bound substrates.Citation46 Arabidopsides were discussed to function as a storage pool of OPDA, as active agents against bacterial and fungal pathogensCitation47 as well as senescence-promoting compounds.Citation48
There is not mechanistic explanation for OPDA perception, which means existence of a putative OPDA receptor. At least some of the OPDA specific responses might be explained via properties of the so-called reactive electrophile species (RES). This type of compounds carry one α,β-unsaturated carbonyl group like OPDA or the non-enzymatically formed phytoprostanes.Citation49,Citation50 Microarray analyses revealed that these structurally similar cyclopentenone compounds induce the expression of a set of stress-related genes such as glutathione-S-transferase (GST) which differ from those genes which were induced by JA.Citation31 This cyclopentenone-induced gene expression is preferentially dependent on TGAs, a subclass of bZIP transcription factors. The RES-oxylipins like OPDA can be conjugated via GST activity to glutathione thereby affecting the biological activity of OPDA, and even protein modification by OPDA was suggested.Citation31 Such scenario of an OPDA-signaling cascade does not require an “OPDA receptor” but would give specificity for the RES structure of OPDA via conjugation or the modified target proteins. Future work will show how the diversity and COI1-, JA- and OPDA-dependent/independent signaling pathways are organized and how these cascades evolved.
Acknowledgment
The authors work was funded by the Deutsche Forschungsgemeinschaft (SFB 363 project C5 and SFB 648 project C2 to C.W. and project HA2655/12-1 to B.H.) and the Region HANA for Biotechnological and Agricultural Research (grant no. ED0007/01/01 to M.S., Czech Republic).
References
- Demole E, Lederer E, Mercier D. Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant charactéristique de lèssence de jasmin. Helv Chim Acta 1962; 45:675 - 85; http://dx.doi.org/10.1002/hlca.19620450233
- Wasternack C, Forner S, Strnad M, Hause B. Jasmonates in flower and seed development. Biochimie 2012; •••; http://dx.doi.org/10.1016/j.biochi.2012.06.005; PMID: 22705387
- Glauser G, Dubugnon L, Mousavi SAR, Rudaz S, Wolfender J-L, Farmer EE. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 2009; 284:34506 - 13; http://dx.doi.org/10.1074/jbc.M109.061432; PMID: 19846562
- Koo AJK, Gao X, Jones AD, Howe GA. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J 2009; 59:974 - 86; http://dx.doi.org/10.1111/j.1365-313X.2009.03924.x; PMID: 19473329
- Koo AJK, Howe GA. The wound hormone jasmonate. Phytochemistry 2009; 70:1571 - 80; http://dx.doi.org/10.1016/j.phytochem.2009.07.018; PMID: 19695649
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 2008; 177:114 - 27; PMID: 17995915
- Schaller A, Stintzi A. Enzymes in jasmonate biosynthesis - structure, function, regulation. Phytochemistry 2009; 70:1532 - 8; http://dx.doi.org/10.1016/j.phytochem.2009.07.032; PMID: 19703696
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 2007; 100:681 - 97; http://dx.doi.org/10.1093/aob/mcm079; PMID: 17513307
- Heitz T, Widemann E, Lugan R, Miesch L, Ullmann P, Désaubry L, et al. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone Jasmonoyl-isoleucine for catabolic turnover. J Biol Chem 2012; 287:6296 - 306; http://dx.doi.org/10.1074/jbc.M111.316364; PMID: 22215670
- Koo AJ, Howe GA. Catabolism and deactivation of the lipid-derived hormone jasmonoyl-isoleucine. Frontiers Plant Sci 2012; 3.
- Koo AJK, Cooke TF, Howe GA. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-L-isoleucine. Proc Natl Acad Sci U S A 2011; 108:9298 - 303; http://dx.doi.org/10.1073/pnas.1103542108; PMID: 21576464
- Nakamura Y, Mithöfer A, Kombrink E, Boland W, Hamamoto S, Uozumi N, et al. 12-hydroxyjasmonic acid glucoside is a COI1-JAZ-independent activator of leaf-closing movement in Samanea saman. Plant Physiol 2011; 155:1226 - 36; http://dx.doi.org/10.1104/pp.110.168617; PMID: 21228101
- Matthes MC, Bruce TJ, Ton J, Verrier PJ, Pickett JA, Napier JA. The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta 2010; 232:1163 - 80; http://dx.doi.org/10.1007/s00425-010-1244-4; PMID: 20711606
- Ziegler J, Stenzel I, Hause B, Maucher H, Hamberg M, Grimm R, et al. Molecular cloning of allene oxide cyclase. The enzyme establishing the stereochemistry of octadecanoids and jasmonates. J Biol Chem 2000; 275:19132 - 8; http://dx.doi.org/10.1074/jbc.M002133200; PMID: 10764787
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci U S A 2001; 98:12837 - 42; http://dx.doi.org/10.1073/pnas.211311098; PMID: 11592974
- Böttcher C, Pollmann S. Plant oxylipins: plant responses to 12-oxo-phytodienoic acid are governed by its specific structural and functional properties. FEBS J 2009; 276:4693 - 704; http://dx.doi.org/10.1111/j.1742-4658.2009.07195.x; PMID: 19663904
- Göbel C, Feussner I. Methods for the analysis of oxylipins in plants. Phytochemistry 2009; 70:1485 - 503; http://dx.doi.org/10.1016/j.phytochem.2009.07.040; PMID: 19735927
- Hause B, Stenzel I, Miersch O, Maucher H, Kramell R, Ziegler J, et al. Tissue-specific oxylipin signature of tomato flowers: allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles. Plant J 2000; 24:113 - 26; http://dx.doi.org/10.1046/j.1365-313x.2000.00861.x; PMID: 11029709
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling H-Q, et al. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 2003; 15:1646 - 61; http://dx.doi.org/10.1105/tpc.012237; PMID: 12837953
- Browse J. The power of mutants for investigating jasmonate biosynthesis and signaling. Phytochemistry 2009; 70:1539 - 46; http://dx.doi.org/10.1016/j.phytochem.2009.08.004; PMID: 19740496
- Stintzi A, Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci U S A 2000; 97:10625 - 30; http://dx.doi.org/10.1073/pnas.190264497; PMID: 10973494
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010; 468:400 - 5; http://dx.doi.org/10.1038/nature09430; PMID: 20927106
- Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 1998; 280:1091 - 4; http://dx.doi.org/10.1126/science.280.5366.1091; PMID: 9582125
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 2004; 16:126 - 43; http://dx.doi.org/10.1105/tpc.017954; PMID: 14688297
- Goetz S, Hellwege A, Stenzel I, Kutter C, Hauptmann V, Forner S, et al. Role of cis-12-oxo-phytodienoic acid in tomato embryo development. Plant Physiol 2012; 158:1715 - 27; http://dx.doi.org/10.1104/pp.111.192658; PMID: 22337921
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, et al. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 2009; 5:344 - 50; http://dx.doi.org/10.1038/nchembio.161; PMID: 19349968
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007; 448:666 - 71; http://dx.doi.org/10.1038/nature06006; PMID: 17637675
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007; 448:661 - 5; http://dx.doi.org/10.1038/nature05960; PMID: 17637677
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 2009; 21:2220 - 36; http://dx.doi.org/10.1105/tpc.109.065730; PMID: 19717617
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci U S A 2008; 105:7100 - 5; http://dx.doi.org/10.1073/pnas.0802332105; PMID: 18458331
- Mueller S, Hilbert B, Dueckershoff K, Roitsch T, Krischke M, Mueller MJ, et al. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 2008; 20:768 - 85; http://dx.doi.org/10.1105/tpc.107.054809; PMID: 18334669
- Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 2005; 139:1268 - 83; http://dx.doi.org/10.1104/pp.105.067058; PMID: 16258017
- Godt DE, Roitsch T. Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol 1997; 115:273 - 82; http://dx.doi.org/10.1104/pp.115.1.273; PMID: 9306701
- Zanor MI, Osorio S, Nunes-Nesi A, Carrari F, Lohse M, Usadel B, et al. RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol 2009; 150:1204 - 18; http://dx.doi.org/10.1104/pp.109.136598; PMID: 19439574
- Jin Y, Ni D-A, Ruan Y-L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 2009; 21:2072 - 89; http://dx.doi.org/10.1105/tpc.108.063719; PMID: 19574437
- Stelmach BA, Müller A, Hennig P, Laudert D, Andert L, Weiler EW. Quantitation of the octadecanoid 12-oxo-phytodienoic acid, a signalling compound in plant mechanotransduction. Phytochemistry 1998; 47:539 - 46; http://dx.doi.org/10.1016/S0031-9422(97)00547-5; PMID: 9461672
- Blechert S, Bockelmann C, Füßlein M, von Schrader T, Stelmach B, Niesel U, et al. Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta 1999; 207:470 - 9; http://dx.doi.org/10.1007/s004250050506
- Ribot C, Zimmerli C, Farmer EE, Reymond P, Poirier Y. Induction of the Arabidopsis PHO1;H10 gene by 12-oxo-phytodienoic acid but not jasmonic acid via a CORONATINE INSENSITIVE1-dependent pathway. Plant Physiol 2008; 147:696 - 706; http://dx.doi.org/10.1104/pp.108.119321; PMID: 18434606
- Ralhan A, Schöttle S, Thurow C, Iven T, Feussner I, Polle A, et al. The vascular pathogen Verticillium longisporum requires a jasmonic acid-independent COI1 function in roots to elicit disease symptoms in Arabidopsis shoots. Plant Physiol 2012; 159:1192 - 203; http://dx.doi.org/10.1104/pp.112.198598; PMID: 22635114
- Köster J, Thurow C, Kruse K, Meier A, Iven T, Feussner I, et al. Xenobiotic- and jasmonic acid-inducible signal transduction pathways have become interdependent at the Arabidopsis CYP81D11 promoter. Plant Physiol 2012; 159:391 - 402; http://dx.doi.org/10.1104/pp.112.194274; PMID: 22452854
- Stumpe M, Göbel C, Faltin B, Beike AK, Hause B, Himmelsbach K, et al. The moss Physcomitrella patens contains cyclopentenones but no jasmonates: mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology. New Phytol 2010; 188:740 - 9; http://dx.doi.org/10.1111/j.1469-8137.2010.03406.x; PMID: 20704658
- Robson F, Okamoto H, Patrick E, Harris S-R, Wasternack C, Brearley C, et al. Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 2010; 22:1143 - 60; http://dx.doi.org/10.1105/tpc.109.067728; PMID: 20435902
- Brüx A, Liu T-Y, Krebs M, Stierhof Y-D, Lohmann JU, Miersch O, et al. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell 2008; 20:1088 - 100; http://dx.doi.org/10.1105/tpc.108.058362; PMID: 18441211
- Dave A, Hernández ML, He Z, Andriotis VME, Vaistij FE, Larson TR, et al. 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 2011; 23:583 - 99; http://dx.doi.org/10.1105/tpc.110.081489; PMID: 21335376
- Dave A, Graham IA. Oxylipin signalling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA). Frontiers Plant Sci 2012; 3:42
- Nilsson AK, Fahlberg P, Ellerström M, Andersson MX. Oxo-phytodienoic acid (OPDA) is formed on fatty acids esterified to galactolipids after tissue disruption in Arabidopsis thaliana. FEBS Lett 2012; 586:2483 - 7; http://dx.doi.org/10.1016/j.febslet.2012.06.010; PMID: 22728240
- Kourtchenko O, Andersson MX, Hamberg M, Brunnström A, Göbel C, McPhail KL, et al. Oxo-phytodienoic acid-containing galactolipids in Arabidopsis: jasmonate signaling dependence. Plant Physiol 2007; 145:1658 - 69; http://dx.doi.org/10.1104/pp.107.104752; PMID: 17951463
- Hisamatsu Y, Goto N, Hasegawa K, Shigemori H. Senescence-promoting effect of arabidopside A. Z Naturforsch C 2006; 61:363 - 6; PMID: 16869494
- Alméras E, Stolz S, Vollenweider S, Reymond P, Mène-Saffrané L, Farmer EE. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J 2003; 34:205 - 16; http://dx.doi.org/10.1046/j.1365-313X.2003.01718.x; PMID: 12694595
- Farmer EE, Davoine C. Reactive electrophile species. Curr Opin Plant Biol 2007; 10:380 - 6; http://dx.doi.org/10.1016/j.pbi.2007.04.019; PMID: 17646124