Abstract
Plants respond to herbivory through various morphological, biochemicals, and molecular mechanisms to counter/offset the effects of herbivore attack. The biochemical mechanisms of defense against the herbivores are wide-ranging, highly dynamic, and are mediated both by direct and indirect defenses. The defensive compounds are either produced constitutively or in response to plant damage, and affect feeding, growth, and survival of herbivores. In addition, plants also release volatile organic compounds that attract the natural enemies of the herbivores. These strategies either act independently or in conjunction with each other. However, our understanding of these defensive mechanisms is still limited. Induced resistance could be exploited as an important tool for the pest management to minimize the amounts of insecticides used for pest control. Host plant resistance to insects, particularly, induced resistance, can also be manipulated with the use of chemical elicitors of secondary metabolites, which confer resistance to insects. By understanding the mechanisms of induced resistance, we can predict the herbivores that are likely to be affected by induced responses. The elicitors of induced responses can be sprayed on crop plants to build up the natural defense system against damage caused by herbivores. The induced responses can also be engineered genetically, so that the defensive compounds are constitutively produced in plants against are challenged by the herbivory. Induced resistance can be exploited for developing crop cultivars, which readily produce the inducible response upon mild infestation, and can act as one of components of integrated pest management for sustainable crop production.
Introduction
Plants and insects have been living together for more than 350 million years. In co- evolution, both have evolved strategies to avoid each other’s defense systems. This evolutionary arms race between plants and insects has resulted in the development of an elegant defense system in plants that has the ability to recognize the nonself molecules or signals from damaged cells, much like the animals, and activates the plant immune response against the herbivores.Citation1-Citation3 To counter the herbivore attack, plants produce specialized morphological structures or secondary metabolites and proteins that have toxic, repellent, and/or antinutitional effects on the herbivores.Citation4-Citation6 Plants confront the herbivores both directly by affecting host plant preference or survival and reproductive success (direct defense), and indirectly through other species such as natural enemies of the insect pests (indirect defense).Citation1,Citation7,Citation8 Direct defenses are mediated by plant characteristics that affect the herbivore’s biology such as mechanical protection on the surface of the plants (e.g., hairs, trichomes, thorns, spines, and thicker leaves) or production of toxic chemicals such as terpenoids, alkaloids, anthocyanins, phenols, and quinones) that either kill or retard the development of the herbivores.Citation9 Indirect defenses against insects are mediated by the release of a blend of volatiles that specifically attract natural enemies of the herbivores and/or by providing food (e.g., extra floral nectar) and housing to enhance the effectiveness of the natural enemies.Citation8 Research on plant-herbivore interactions is one of the most important and multidisciplinary undertakings in plant biology involving various disciplines to describe chemical and ecological processes influencing the outcome of plant - herbivore interactions. Our understanding of how plants communicate with their neighbors, symbionts, pathogens, herbivores, and with their personal “bodyguards”- the natural enemies, both above and below ground, via chemical signals, is still in its infancy. This is an enthralling area from an ecological point of view, and has a great potential for utilization in crop protection. Understanding the nature of gene expression of the plant defensive traits will have a tremendous application in designing crop plants with better protection against the herbivores. This in turn will reduce the need for use of harmful pesticides for insect control. However, the arms race between plants and herbivores will continue, and herbivores could co-evolve in response to the resistant plant genotypes. Knowledge of the complex chemical plant-herbivore interactions is required to optimize the production of new crops.
Host plant defenses against insects
Plants respond to herbivore attack through an intricate and dynamic defense system that includes structural barriers, toxic chemicals, and attraction of natural enemies of the target pests ().Citation1,Citation9,Citation10 Both defense mechanisms (direct and indirect) may be present constitutively or induced after damage by the herbivores. Induced response in plants is one of the important components of pest control in agriculture, and has been exploited for regulation of insect herbivore population.Citation1,Citation11,Citation12 Over the past few decades, considerable progress has been made in studying induced responses in plants against different stresses, and has become an important topic in evolutionary biology and ecology. Although induced responses have some metabolic costs,Citation13 they are very important when aimed at alleviating the stress of immediate concern, as most of these chemicals are produced in response to herbivore attack.Citation14,Citation15 Induced defenses make the plants phenotypically plastic, and thereby, decrease the chances of the attacking insects to adapt to the induced chemicals.Citation1,Citation12
Figure 1. Mechanism of induced resistance in plants. POD, peroxidase; PPO, polyphenol oxidase; PAL, phenylalanine ammonia lyase; TAL, tyrosine alanine ammonia lyase; LOX, lipoxygenase; SOD, superoxide dismutase; APX, ascorbate peroxidase; HIPVs, Herbivore induced plant volatiles
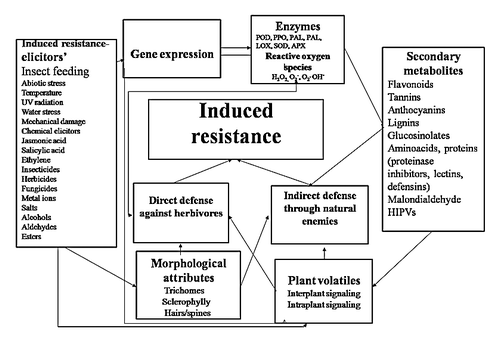
Changes in defensive constituents of a plant on account of insect attack develop unpredictability in the plant environment for insect herbivores, which in turn, affects the fitness and behavior of the herbivores.Citation5,Citation6,Citation14 If induced response occurs very early, it is of great benefit to the plant, and reduces the subsequent herbivore and pathogen attack, besides improving overall fitness of the plant.Citation12 Plants with high variability in defensive chemicals exhibit a better defense compared with those with moderate variability.Citation5,Citation6 Progress in insect-plant interactions has improved our understanding of the evolution of defensive approaches exploited deployed by the plants against herbivory;Citation10 however, the underlying mechanisms of defense are less clearly understood
Direct defenses
Plant structural traits such as leaf surface wax, thorns or trichomes, and cell wall thickness/ and lignification form the first physical barrier to feeding by the herbivores, and the secondary metabolites such act as toxins and also affect growth, development, and digestibility reducers form the next barriers that defend the plant from subsequent attack.Citation9,Citation16 Moreover, synergistic effect among different defensive components enhances the defensive system of plants against the herbivores invaders. In tomato, alkaloids, phenolics, proteinase inhibitors (PIs), and the oxidative enzymes when ingested separately result in a reduced affect, but act together in a synergistic manner, affecting the insect during ingestion, digestion and metabolism.Citation17 In Nicotiana attenuata (Torr. ex Watson), trypsin proteinase inhibitors and nicotine expression, contributed synergistically to the defensive response against Spodoptera exigua (Hub.).Citation15 The role of morphological and biochemical constituents in host plant resistance (HPR), and induced responses to insect damage will be discussed below.
Morphological structures
Plant structures are the first line of defense against herbivory, and play an important role in host plant resistance (HPR) to insects. The first line of plant defense against insect pests is the erection of a physical barrier either through the formation of a waxy cuticle,Citation9,Citation16 and/or the development of spines, setae, and trichomes.Citation18,Citation19 Structural defenses includes morphological and anatomical traits that confer a fitness advantage to the plant by directly deterring the herbivores from feeding,Citation16 and range from prominent protrubances on a plant to microscopic changes in cell wall thickness as a result of lignification and suberization.Citation9,Citation19 Structural traits such as spines and thorns (spinescence), trichomes (pubescence), toughened or hardened leaves (sclerophylly), incorporation of granular minerals into plant tissues, and divaricated branching (shoots with wiry stems produced at wide axillary angles) play a leading role in plant protection against herbivory.Citation9,Citation19,Citation20 Sclerophylly refers to the hardened leaves, and plays an active role in plant defense against herbivores by reducing the palatability and digestibility of the tissues, thereby, reducing the herbivore damage.Citation9,Citation21
Spinescence includes plant structures such as spines, thorns and prickles. It has been reported to defend the plants against many insects.Citation9 Pubescence consists of the layer of hairs (trichomes) extending from the epidermis of the above ground plant parts including stem, leaves, and even fruits, and occur in several forms such as straight, spiral, stellate, hooked, and glandular.Citation9 Chamarthi et al.Citation20 reported that leaf glossiness, plumule and leaf sheath pigmentation were responsible for shoot fly Atherigona soccata (Rondani) resistance in sorghum Sorghum bicolor (L.) (Moench).
Trichomes
Trichomes play an imperative role in plant defense against many insect pests and involve both toxic and deterrent effects.Citation20,Citation21 Trichome density negatively affects the ovipositional behavior, feeding and larval nutrition of insect pests.Citation21 In addition, dense trichomes affect the herbivory mechanically, and interfere with the movement of insects and other arthropods on the plant surface, thereby, reducing their access to leaf epidermis.Citation16 These can be, straight, spiral, hooked, branched, or un-branched and can be glandular or nonglandular.Citation9 Glandular trichomes secrete secondary metabolites including flavonoids, terpenoids, and alkaloids that can be poisonous, repellent, or trap insects and other organisms, thus forming a combination of structural and chemical defense.Citation9,Citation18
Induction of trichomes in response to insect damage has been reported in many plants.Citation22 This increase in trichome density in response to damage can only be observed in leaves developing during or subsequent to insect attack, since the density of trichomes of existing leaves does not change.Citation16 Dalin and BjorkmanCitation23 reported that damage by adult leaf beetles, Phratora vulgatissima L. in Salix cinerea L. induced higher trichome density in the new leaves developing thereafter. Likewise, increase in trichome density in S. cinera in response to coleopteran damage has also been reported.Citation24 Increase in trichome density after insect damage has also been reported in Lepidium virginicum L. and Raphanus raphanistrum L.Citation22 In black mustard, trichomes density and glucosinolate levels were elevated after feeding by Pieris rapae (L.).Citation25 Trichome density increased in Alnus incana Moench as a result of damage by beetles.Citation26 The increase in trichome density in response to herbivory is typically between 25 to 100%, however, there are cases where 500 – 1000% increase in trichome density has also been reported. Changes in trichome density occur within days or weeks after insect damage.Citation22-Citation24 Furthermore, change in relative proportion of glandular and non-glandular trichomes is also induced by herbivory.Citation22 A positive correlation has been observed between natural enemies’ abundance and trichome density. Trichome exudates also serve as extra floral nectar (EFN) for scelonid egg parasitoid, of squash bugs, Gryon pennsylvanicum.Citation27
Secondary metabolites and plant defense
Secondary metabolites are the compounds that do not affect the normal growth and development of a plant, but reduce the palatability of the plant tissues in which they are produced.Citation1 The defensive (secondary) metabolites can be either constitutive stored as inactive forms or induced in response to the insect or microbe attack. The former are known as phytoanticipins and the latter as phytoalexins. The phytoanticipins are mainly activated by β-glucosidase during herbivory, which in turn mediate the release of various biocidal aglycone metabolites.Citation28 The classic examples of phytoanticipins are glucosinolates that are hydrolyzed by myrosinases (endogenous β-thioglucoside glucohydrolases) during tissue disruption. Other phytoanticipins include Benzoxazinoids (BXs), which are widely distributed among Poaceae. Hydrolyzation of BX-glucosides by plastid-targeted β-glucosidases during tissue damage leads to the production of biocidal aglycone BXs, which play an important role in plant defense against insects.Citation28 Phytoalexins include isoflavonoids, terpenoids, alkaloids, etc., that influence the performance and survival of the herbivores.Citation29 The secondary metabolites not only defend the plants from different stresses, but also increase the fitness of the plants. It has been reported that maize HPR to corn earworm, Helicoverpa zea (Boddie) is mainly due to the presence of the secondary metabolites C-glycosyl flavone maysin [2”- O - a –L-rhamnosyl- 6- C - (6-deoxy- xylo -hexos-4-ulosyl) luteolin] and the phenylpropanoid product, chlorogenic acid.Citation30 Compound, 4, 4- dimethyl cyclooctene has been found to be responsible for shoot fly A. soccata resistance in sorghum S. bicolor.Citation31
Secondary metabolites have been primarily studied as the mediators of direct defense, however much is to be done to reveal the unidentified or emerging signaling pathways. Mass spectrometry used for the secondary metabolite profiling and gene expression analysis by high-throughput sequencing has made this field more exciting and cost-effective. Study on secondary metabolites could lead to the identification of new signaling molecules involved in plant resistance against herbivores and other stresses. Ultimately genes and enzymes involved in the biosynthesis of these metabolites could be identified. Role of some of the secondary metabolites in plant defense will be discussed below.
Plant phenolics
Among the secondary metabolites, plant phenols constitute one of the most common and widespread group of defensive compounds, which play a major role in HPR against herbivores, including insects.Citation4-Citation6,Citation18 Phenols act as a defensive mechanism not only against herbivores, but also against microorganisms and competing plants. Qualitative and quantitative alterations in phenols and elevation in activities of oxidative enzyme in response to insect attack is a general phenomenon.Citation5,Citation6,Citation32
Lignin, a phenolic heteropolymer plays a central role in plant defense against insects and pathogens.Citation32 It limits the entry of pathogens by blocking physically or increasing the leaf toughness that reduces the feeding by herbivores, and also decreases the nutritional content of the leaf.Citation33 Lignin synthesis has been found to be induced by herbivory or pathogen attack and its rapid deposition reduce further growth of the pathogen or herbivore fecundity.Citation33 Increase in expression of lignin associated genes (CAD/CAD-like genes) in plants infected with pests and pathogens have been documented.Citation32
Oxidation of phenols catalyzed by polyphenol oxidase (PPO) and peroxidase (POD) is a potential defense mechanism in plants against herbivorous insects. Quinones formed by oxidation of phenols bind covalently to leaf proteins, and inhibit the protein digestion in herbivores.Citation34 In addition, quinones also exhibit direct toxicity to insects.Citation17,Citation34 Alkylation of amino acids reduces the nutritional value of plant proteins for insects, which in turn negatively affects the insect growth and development.Citation34 Phenols also play an important role in cyclic reduction of reactive oxygen species (ROS) such as superoxide anion and hydroxide radicals, H2O2, and singlet oxygen, which in turn activate a cascade of reactions leading to the activation of defensive enzymes.Citation35 Simple phenolics (salicylates) act as antifeedant to insect herbivores such as Operophtera brumata (L.) in Salix leaves, and there is a negative correlation between the salicylate levels and the larval growth, however, salicylic acid (SA) is much more important as phytohormone than as deterrent.Citation36
Flavonoids
Flavonoids play a central role in various facets of plant life especially in plant-environment interactions.Citation37 These defend plants against various biotic and abiotic stresses including UV radiations, pathogens and insect pests.Citation37 Flavonoids are cytotoxic and interact with different enzymes through complexation. Both flavonoids and isoflavonoids protect the plant against insect pests by influencing the behavior, and growth and development of insects.Citation36 In addition, flavonoids scavenge the free radicals including ROS, and reduce their formation by chelating the metals.Citation37 Flavonoids are divided into various classes that include anthocyanins, flavones, flavonols, flavanones, dihydroflavonols, chalcones, aurones, flavan, and proanthocyanidins.Citation37 More than 5,000 flavonids have been reported in plants. A number of flavones such as flavonols, flavones, proanthocyanidins, flavan 3-ols, flavonones, flavans, and isoflavonoids have been investigated as feeding deterrents against many insect pests. Flavonoids such as flavones 5 -hydroxyisoderricin,7- methoxy-8- (3- methylbutadienyl) –flavanone and 5-methoxyisoronchocarpin isolated from Tephrosia villosa (L.), T. purpurea (L.), and T. vogelii Hook, respectively have been found as feeding deterrents against Spodoptera exempta (Walk.), and Spodoptera littoralis Bios.Citation38 Overexpressing a transcription factor controlling flavonoid production in Arabidopsis has been reported to confer resistance against Spodoptera frugiperda (J.E. Smith).Citation39 Angustone A, licoisoflavone B, angustone B, and angustone C. Isoflavones, licoisoflavone A, luteone, licoisoflavone B, and wighteone have been found to be not only feeding deterrents to insects, but also have antifungal activity against the fungi, Colletotrichum gloeosporiode (Penz.) and Cladosporium cladosporioides (Fres.).Citation40 Isoflavonoids (judaicin, judaicin-7-O-glucoside, 2-methoxyjudaicin, and maackiain) isolated from the wild relatives of chickpea act as antifeedant against Helicoverpa armigera (Hubner) at 100 ppm. Judaicin and maackiain were also found to be deterrent to S. littoralis and S. frugiperda, respectively.Citation41 Cyanopropenyl glycoside and alliarinoside strongly inhibit feeding by the native American butterfly, Pieris napi oleracea L., while a flavone glycoside, isovitexin-6”-D-β-glucopyranoside acts as a direct feeding deterrent to the late instars.Citation42
Tannins
Tannins have a strong deleterious effect on phytophagous insects and affect the insect growth and development by binding to the proteins, reduce nutrient absorption efficiency, and cause midgut lesions.Citation18,Citation43,Citation44 Tannins are astringent (mouth puckering) bitter polyphenols and act as feeding deterrents to many insect pests. They precipitate proteins nonspecifically (including the digestive enzymes of herbivores), by hydrogen bonding or covalent bonding of protein –NH2 groups. In addition, tannins also chelate the metal ions, thereby reducing their bioavailability to herbivores. When ingested, tannins reduce the digestibility of the proteins thereby decrease the nutritive value of plants and plant parts to herbivores. Role of tannins in plant defense against various stresses and their induction in response to insect damage has been studied in many plants.Citation44 For example, e.g., in Populus species,Citation45 and in Pinus sylvestris L.Citation46 However, no effect of herbivore damage on tannin content was observed in Quercus serrata (Thunb.)Citation47 and Betula pendula Roth.Citation48 Like proteinase inhibitors and oxidative enzymes, tannins have been reported to be systemically induced in neighboring leaves of the damaged plant.Citation45
Condensed tannins are oligomeric or polymeric flavonoids, also known as proanthocyanidins. They have diverse structures and functions. They act as feeding deterrents against some insects such as, Lymantria dispar (L.), Euproctis chrysorrhoea (L.) and O. brumata.Citation49,Citation50 Condensed tannins such as (+) -catechin, (+) - gallocatechin, and vanillin in leaves of Quercus robur L. inhibited winter moth larvae, O. brumata.Citation49 Procyanindin polymers have been found as feeding deterrent to Aphis Craccivora (Koch) in groundnut.Citation51 Condensed tannins from Alaska paper birch (coated on birch leaves at 3% dry wt.) reduced the pupal mass and survival of Rheumaptera hastata (L.) larvae.Citation52 It has been reported that induction of tannins in Populus tremuloides Michx. leaves in response to wound- and herbivore occur by transcriptional activation of the flavonoid pathway.Citation45 Genes responsible for the production of tannins in response to wounding have been identified and are activated by the expression of a condensed tannins regulatory gene, PtMYB134, which is itself induced by damage.Citation53 Furthermore, induction of tannin is also stimulated by light stress,Citation14,Citation53 and exposure to UV light in hybrid poplar.Citation53 However, some polyphagous insect species have the ability to tolerate gallotannins, e.g., Shistocerca gregaria (Forsk.) tolerates tannins by hydrolyzing them rapidly to avoid any damaging effects by restricting the passage of tannins by adsorbing them on the thick peritrophic membrane, and by inhibiting the tannin protein complex formation by surfactants in the midgut.Citation54
Plant defensive proteins
Ecologically, in insect-plant interaction, interrelationship between two is important for the survival of the both. Insects always look for a true and healthy host plant that can provide them proper food and could be suitable for mating, oviposition and also provides food for the offsprings. The nutritional requirements of insects are similar to other animals, and any imbalance in digestion and utilization of plant proteins by the insects’ results in drastic effects on insect physiology. Alteration of gene expression under stress including insect attack leads to qualitative and quantitative changes in proteins, which in turn play an important role in signal transduction, and oxidative defense ().Citation4,Citation55 Many plant proteins ingested by insects are stable, and remain intact in the midgut, and also move across the gut wall into the hemolymph. An alteration in the protein’s amino acid content or sequence influences the function of that protein. Likewise, anti-insect activity of a proteolysis-susceptible toxic protein can be improved by administration of protease inhibitors (PIs), which prevent degradation of the toxic proteins, and allows them to exert their defensive function. Better understanding of protein structure and post-translational modifications contributing to stability in the herbivore gut would assist in predicting toxicity and mechanism of plant resistance proteins (PRPs). Recent advances in microarray and proteomic approaches have revealed that a wide spectrum of PRPs is involved in plant defense against herbivores.Citation56,Citation57 Due to diverse feeding habits of arthropods, multiple signaling pathways including jasmonic acid (JA), SA and/or ethylene (ET) regulate arthropod-inducible proteins.Citation8
Table 1. Plant defensive proteins against insect pests
Plant lectins
Lectins are carbohydrate-binding (glyco) proteins, ubiquitous in nature, and have protective function against a range of pests.Citation58,Citation59 The insecticidal activities of different plant lectins have been utilized as naturally occurring insecticides against insect pests ().Citation60 One of the most important properties of lectins is their survival in the digestive system of herbivores that gives them a strong insecticidal potential.Citation59 They act as antinutritive and/or toxic substances by binding to membrane glycosyl groups lining the digestive tract, leading to an array of harmful systemic reactions.Citation58,Citation59 Lectins are stable over a large range of pH and damage the luminal epithelial membranes, thereby interfere with the nutrient digestion and absorption.Citation58 Disruption of lipid, carbohydrate, and protein metabolism causes enlargement and/or atrophy of key tissues, which in turn alters the hormonal and immunological status, threatening the growth and development of insects.Citation58-Citation60
Table 2. Plant defensive lectins and lectin like proteins and target insect pests
Lectins have been found to be promising against homopteran,Citation58,Citation60 lepidopteran,Citation61 and coleopteran insects.Citation61 Insecticidal properties of Galanthus nivalis L. agglutinin (GNA) were the first plant lectin shown to be active against hemipteran insects.Citation62 Efficacies of carbohydrate binding plant lectins such as GNA, Phaseolus haemagglutinin, and wheat germ agglutinin, have been studied in detail against many insect pests.Citation59 Mannose - binding lectins have been reported to be effective against sucking insects, because of their interaction with a specific carbohydrate residue of the cell membrane.Citation60 Expression of lectin coding genes in transgenic plants and their defense against insects has been worked out in many plants, e.g., GNA, PSA (Pisum sativum L.; pea), WGA (Triticum vulgare Kunth; wheatgerm), ConA (Canavalia ensiformis (L.); jack bean), AIA (Artocarpus integrifolia Forst.; jack fruit), OSA (Oryza sativa L.; rice), ASAL (Allium sativum L.), and UDA (Urtica dioica L.; stinging nettle).Citation58,Citation60,Citation63 The Arum maculatum lectin has been found effective against the aphids Lipaphis erysimi (Kalt.) and A. craccivora when incorpoated in an artificial diet.Citation64
Studies on the mechanism of action of the mannose-specific lectin, GNA against brown planthopper (Nilaparvata lugens (Stal.) in rice has shown that that GNA binds to the luminal surface of the midgut epithelial cells within the planthopper by recognizing the cell surface carbohydrate moieties of glycoproteins and/or other glycoconjugates in the gut.Citation65 Immuno-labeling GNA assay has shown its presence in the fat bodies, ovarioles, and hemolymph, indicating the ability of GNA to cross the midgut epithelial barrier and pass into the insect’s circulatory system leading to the systemic toxic effect.Citation65 Partial resistance to homopteran insect pests has been reported in transgenic plants expressing snowdrop lectin in tobacco,Citation66 rice,Citation67 and wheat.Citation62
Plant lectins are induced by elicitors as an induced response to various stresses.Citation68 JA induced the expression of NICTABA lectin in tobacco leaves.Citation68,Citation69 Induction of NICTABA by herbivores infestation including S. littoralis, Manduca sexta L. and Tetranychus urticae Koch has been reported in tobacco plants.Citation69 Expression of a mannose-binding jacalin-like lectin called Hessian fly, Mayetiola destructor (Say) responsive protein 1 (HFR1), and two chimerolectin- like proteins called HFR2 and HFR3 have been reported to be induced by the larvae of Hessian fly, M. destructor in wheat.Citation70,Citation71 Differences in feeding behavior of insects results in expression of different lectins, e.g., larvae of the fall armyworm, S. frugiperda induced HFR2, but not HFR3 expression while the phloem-feeding bird cherry-oat aphid, Rhopalosiphum padi Koch, induced HFR3 and HFR2, but latter was expressed much later (12 d) than the former (24 h).Citation70,Citation72 Several jasmonate-inducible lectins are expressed in leaf tissues of monocots such as rice, barley, wheat, rye, and maize.Citation73 Advancement of our understanding in induction of plant lectins in response to various stresses, especially herbivory, and their role in plant defense has the potential for utilization of these entomotoxic lectins in crop protection through genetic engineering. Although, transformation of lectin genes into plants seems to be very attractive and effective, care is needed, because of possible toxicity of some lectins to non-target organisms, including mammals.
Proteinase inhibitors
Proteinase inhibitors (PIs) cover one of the most abundant defensive classes of proteins in plants. Higher concentration of PIs occurs in storage organs such as seeds and tubers, and 1 to 10% of their total proteins comprise of PIs, which inhibit different types of enzymes and play an important role in plant defense against insect herbivory ().Citation74,Citation75 PIs bind to the digestive enzymes in the insect gut and inhibit their activity, thereby reduce protein digestion, resulting in the shortage of amino acids, and slow development and/or starvation of the insects.Citation76 The defensive function of many PIs against insect pests, directly or by expression in transgenic plants to improve plant resistance against insects has been studied against many lepidopteran,Citation75 and hemipteran insects.Citation76 The success of transgenic crops in expressing PIs against insect pests has accentuated the need to understand the mechanisms, and interactions of multiple PIs with other defenses, and the adaptive responses of the herbivores.
Many classes of PIs are induced in plants in response to stresses. Kunitz proteinase inhibitors (KPIs) are the serine PIs (SPIs), which are among the most strongly upregulated defense genes in response to wounding or herbivore feeding in plants.Citation14 The SPIs from Solanum nigrum L. has been found to adversely affect a number of insect pests.Citation77 Progress in genome sequencing has resulted in identification of a large number of proteinase inhibitors and other defense components induced in plants on account of herbivore damage. Although most of the KPIs in plants are upregulated in response to insect herbivory, their degree of induction varies as per the insect plant interaction. Various KPIs allow plants to deal with multiple generations of insects by providing a genetic storehouse of varied PIs. However, some insects respond to PIs by constitutive or induced production of PI-insensitive proteases or by inactivation of ingested PIs, thereby, preventing them from binding to sensitive proteases.Citation78 Such a feeding response by insects negatively affects the PI activity, and may result in even greater damage to the plants.Citation15 This counter defense by the insects is a major hindrance to manipulation and utilization of PIs for a longer-lasting plant defense, and there is a need to understand the mechanisms by which insects counteract the PI-based plant defense.
Enzymes
One of the important aspects of HPR against insects is the disruption of insect’s nutrition. The enzymes that impair the nutrient uptake by insects through formation of electrophiles includes peroxidases (PODs), polyphenol oxidases (PPOs), ascorbate peroxidases, and other peroxidases by oxidizing mono- or dihydroxyphenols, that lead to the formation of reactive o-quinones, which in turn polymerize or form covalent adducts with the nucleophilic groups of proteins due to their electrophilic nature (e.g., -SH or e-NH2 of Lys).Citation34,Citation55,Citation79 Other important antioxidative enzymes include lipoxygenases, phenylalanine ammonia lyase, superoxide dismutase, etc. Induction of antioxidative enzymes in plants following herbivory has received considerable attention in recent years.Citation4-Citation6,Citation55-Citation59
Peroxidases (POD)
Oxidative state of the host plants has been associated with HPR to insects,Citation19,Citation80 which results in production of ROS, that are subsequently eliminated by antioxidative enzymes. POD constitutes one such group of enzymes, which scavenges the ROS besides having other defensive roles. PODs are an important component of the immediate response of plants to insect damage.Citation4,Citation5,Citation55 PODs are monomeric hemoproteins distributed as soluble, membrane-bound, and cell wall-bound within the cells, and are widely spread in plants and include several isozymes whose expression depends on tissue, developmental stage, and environmental stimuli.Citation19,Citation55 A number of process are regulated by PODs that have direct or indirect role in plant defense, including lignification, suberization, somatic embryogenesis, auxin metabolism, and wound healing.Citation19,Citation81,Citation82 Role of PODs in plant resistance to insect pests has been studied in various plant systems().Citation5,Citation6,Citation55 Production of phenoxy and other oxidative radicals by the PODs in association with phenols directly deters the feeding by insects and/ or produces toxins that reduce the plant digestibility, which in turn leads to nutrient deficiency in insects with drastic effects on their growth and development.Citation57,Citation83 In addition, PODs have been reported to have direct toxicity in guts of herbivores.Citation78 PODs have been purified and characterized from many plants where they were induced in response to insect attack.Citation19,Citation55,Citation84
Polyphenol oxidases (PPO)
The PPOs are important enzymes in plants that regulate feeding, growth, and development of insect pests, and play a leading role in plant defense against the biotic and abiotic stresses.Citation19,Citation34 PPOs can function in following ways: a) PPO-generated quinones could alkylate essential amino acids, decreasing plant nutritional quality, (b) quinones may produce oxidative stress in the gut lumen through redox cycling, and (c) quinones and ROS produced by phenolic oxidation, could be absorbed and have toxic effects on herbivores. The PPOs are metallo-enzymes that catalyze the oxidation of monophenols and o-diphenols to quinones, which are highly reactive intermediate compounds that readily polymerize, and react with nucleophilic side chain of amino acids and crosslink proteins, thereby reducing the availability of such proteins, and affect the nutritional quality of the food.Citation34,Citation83 Under acidic conditions, quinones form semiquinone radicals that in turn give rise to ROS, while under basic conditions; quinines react with cellular nucleophiles.Citation34 Quinines are more toxic to plant herbivores than the original phenols.Citation34 In addition to their role in digestibility and palatability of plant tissues, melanin formation by PPOs increases the cell wall resistance to insects and pathogens.Citation80 Induction of PPO activity under abiotic and biotic stresses and by treatment with compounds related to the octadecanoid pathway makes it an important tool in plant resistance against different stresses.Citation19,Citation34 The PPO genes are differentially induced by signaling molecules and injury due to wounding, and pathogen, or insect infestation.Citation34,Citation80 Correlation between induction of PPO activity and insect fitness has been reported in many plants including tomato and lettuce.Citation34,Citation82 Although PPOs accumulate in leaves, roots, stems and flowers of the plants, young tissues with greater vulnerability to insect attack exhibit greater induction. The PPOs confer resistance to S. litura, H. armigera, Bemisia tabaci (Gen.), Tetranychus cinnabarinus (Boisd.), Myzus persicae (Sulzer), Empoasca fabae (Harris), Aphis medicaginis (Koch), S. exigua (Hub.), and Agelastica alni (L.).Citation4,Citation5,Citation19,Citation34,Citation85 However, induced PPO levels had no or limited impact on L. dispar, Orgyia leucostigma (JE Smith),Citation86 and Blissus occiduus Barber.Citation81
Lipoxygenases
Lipoxygenases (LOXs) are another group of anti-oxidative enzymes involved in plant defense against many stresses through octadecanoid pathway.Citation87 They catalyze hydroperoxidation of polyunsaturated fatty acids resulting in formation of fatty acid hydroperoxides. The latter are enzymatically and/or chemically degraded to unstable and highly reactive aldehydes, γ-ketols, epoxides,Citation87 and ROS such as hydroxyl radicals, singlet oxygen, superoxide ion and peroxyl, acyl and carbon-centered radicals.Citation35,Citation87 The unstable reactive products interact with proteins resulting in protein-protein cross linking and amino acid damage that in turn affects the amino acid assimilation.Citation35 In addition, lipid peroxidation end products also act as insect repellents or antixenosisCitation87 and are toxic to insect pests (antibiosis).Citation34,Citation35 Major substrates of LOX in plants are linoleic and linolenic acids. One of the most important aspects of LOX in plant defense is the oxidation of linolenic acid in JA signaling pathway, which in turn plays a leading role in activation of plant defense, both directly by production of oxidative enzymes and protease inhibitors,Citation88 and indirectly through the production of volatile organic compounds (VOC) that attract the natural enemies of insect pests.Citation87 Oxygenation of polyunsaturated fatty acids has been found to be catalyzed by LOX, which results in the production of hydroperoxides that are metabolized to compounds such as JA and traumatin.Citation89
Induction of LOX activity in response to herbivory has been studied in many plants such as soybean in response to two-spotted spider mite, T. urticeae,Citation90() in tomato in response to aphids, Macrosiphium euphorbiae Thom., and M. persicae,Citation91 in N. attenuata following infestation by Myzus nicotianae Black.Citation92 and in wheat following Sitobion avenae (F.) infestation.Citation80 The N. attenuata plants deficient in LOX are more vulnerable to attack by M. sexta, which also attract the new herbivores such as Empoasca spp,Citation92 as compared with the plants where LOX3-mediated defense reduced larval growth, food consumption, and frass production.Citation93 Maize plants transformed with the wheat oxalate oxidase gene had upregulation of LOX transcripts and elevation of free phenolics (14-fold), which were positively associated with resistance to the European corn borer, O. nubilalis.Citation88
Indirect defenses
The defensive response in plants to attract natural enemies of herbivores plays a pivotal role in protecting the plants against herbivore attack.Citation7 Indirect defenses can be constitutive or induced as a result of combined action of mechanical damage and elicitors from the attacking herbivore. Production of volatiles and the secretion of extra floral nectar (EFN) mediate interactions of plants with natural enemies of the insect pests (i.e., parasitoids or predators), which actively reduce the numbers of feeding herbivores.Citation7,Citation94 Induced indirect defenses have received increasing attention recently and have been studied on the genetic, biochemical, physiological, and ecological levels.Citation7,Citation8,Citation94
Herbivore induced plant volatiles (HIPVs)
Plants indirectly defend themselves from herbivore feeding by emitting a blend of volatiles and non-volatile compounds. Herbivore-induced plant volatiles (HIPVs) play an important role in plant defense by either attracting the natural enemies of the herbivores or by acting as feeding and/or oviposition deterrent.Citation7,Citation8 HIPVs are the lipophilic compounds with higher vapor pressure which are released from the leaves, flowers, and fruits into the atmosphere, and into the soil from the roots by plants in response herbivore attack.Citation7 The HIPV’s produced vary according to the plant and herbivore species, the developmental stage and condition of the plants and the herbivores.Citation8,Citation94An optimum quantity of volatile compounds is normally released by the plants into the atmosphere, whereas a different blend of volatiles is produced in response to herbivory.Citation8 The volatile blend released by plants in response to insect attack is specific for a particular insect-plant system, including natural enemies and the neighboring plants.Citation8,Citation95 The HIPVs mediate the interactions between plants and arthropods, microorganisms, undamaged neighboring plants, or intraplant signaling that warns undamaged sites within the plant.Citation8,Citation10 Depending upon the modes of feeding of insect pests, different defense signaling pathways are activated, which induce the production of specific volatile compounds.Citation29
The HIPVs include terpenes, green leafy volatiles (GLVs), ethylene, methyl salicylate and other VOCs.Citation7,Citation94 The well-studied metabolites of hydroperoxide lyase (HPL) branch of oxylipin-pathway producing stress-inducible compounds are the GLVs. GLVs are reactive electrophile species involved in stress and defense signals. GLVs consist of C6-aldehydes [(Z)-3-hexenal, n-hexanal] and their respective derivatives such as (Z)-3-hexenol, (Z)-3-hexen-1-yl acetate, and the corresponding E-isomers.Citation8,Citation96 To understand the role of C6-aldehydes and their respective derivatives in plant defense, the GLVs levels have been altered either by application of elicitors,Citation96 or by manipulating genetically the HPL expression in plants. GLVs play an important role in plant defense by attracting natural enemies.Citation3,Citation7,Citation8,Citation96 Plant volatiles such as methyl salicylates and the C16- homoterpene 4, 8, 12- trimethyl-1, 3(E), 7(E), 11- tridecatetraene [(E, E)-TMTT] have been found to attract the predatory mites.Citation97 The most frequent component of the HIPVs is methyl salicylate (MeSA), and has been reported in the headspace of many insect-infested plants including lima bean,Citation8 and Arabidopsis.Citation98 MeSA is a ubiquitous component of many leaf and floral blends and MeSA baited sticky cards attract many insect predators including the big- eyed bug, Geocoris pallens Stal., ladybird beetle, Stethorus punctum picipes (Casey), green lacewing Chrysopa nigricornis Burmeister,Citation99 and other natural enemies.Citation97 Ulland et al.Citation100reported the inhibition of oviposition of cabbage moths Mamestra brassicae L. by MeSA released during infestation, suggesting that MeSA can also be detected by the attacking herbivores. Methyl benzoate (MeBA), which structurally resembles MeSA, has also been detected from insect-infested plants.Citation98 S. frugiperda infestation in rice induces emission of about 30 volatiles, including MeSA and MeBA, which are highly attractant to the natural enemies of S. frugiperda, such as, Cotesia marginiventris (Cresson).Citation101 However, there is an ecological cost of using HIPVs to engineer natural enemies; because HIPVs has the potential of attracting crop pests. For example, Colorado potato beetles, Leptinotarsa decemlineata (Say) is attracted to a blend of volatiles consisting of cis-3-hexenyl acetate, linalool, and MeSA.Citation102
Compounds such as ester methyl salicylate (MeSA), monoterpenes myrcene and β-ocimene, homoterpene (E, E)-4, 8, 12- trimethyltrideca-1, 3, 7, 11-tetraene (TMTT), and sesquiterpene (E, E)-α- farnesene are emitted hours after infestation.Citation7 Systemic release of VOCs is one of the best studied responses specific to herbivores. The HIPVs defend the plants either directly by repelling, deterring and toxicity to the herbivore or indirectly by attracting the natural enemies of the attackers, and thus, protect the plants from further damage.Citation7,Citation94 Lipoxygenase and Shikimic acid pathway metabolites and terpenoid pathway products (terpenoids) play an important role in plant defense, both directly and indirectly.Citation79 Period specific volatile emission has been observed in many plants e.g., lima bean leaves attacked by S. littoralis,Citation103 and hybrid poplar (Populus trichocarpa Torr. and A. Grey X deltoides) leaves infested by forest tent caterpillar, L. dispar emitted a blend of volatiles containing (E)–β- ocimene and other mono-, sesqui- and homoterpenes.Citation104 Maize plants when exposed to (Z)-3- hexanol induced the volatile blend emission that is usually released after caterpillar infestation, and attracts the natural enemies.Citation105
Priming of the volatile emission signals has been reported in many plants.Citation7,Citation106 Engelberth et al.Citation95 reported that application of GLV compounds such as (Z) -3- hexanal, (Z) -3- hexen-1-ol, and (Z) -3- hexenyl acetate individually and blend of volatiles to the maize seedlings enabled the seedlings to respond to wounding and beet armyworm, S. exigua caterpillar regurgitate, and resulted in accumulation of JA and sesquiterpenes as compared with the control plants. Similar observations were recorded by Kessler et al.Citation106 in N. attenuata in response to M. sexta infestation, where low damage was shown by plants primed with clipped sagebrush-released volatiles. Thus, priming plays an important role in plant defense by incomplete turning on of defense related processes to reduce the biochemical investments until the onset of actual attack.Citation95,Citation106 However, there are a few reports where some non-target insect pests were also attracted on account of volatile emission in infested plants, thereby, increasing the insect attack on the plant.Citation107
Transgenic Arabidopsis with overexpression of strawberry nerolidol synthase, a terpene synthase (TPS) responsible for the production of sesquiterpene alcohol (3S)-(E) – nerolidol has been reported to attract the predatory mite, P. persimilis.Citation108 The parasitic wasp, Cotesia marginiventris (Cresson) was attracted to the lepidopteran larvae infesting transgenic maize plants with overexpression of the corn TPS10 gene responsible for the formation of (E)-β- farnesene, (E)-α-bergamotene, and other herbivore induced sesquiterpene hydrocarbons.Citation109
In addition to the plant volatiles released from aerial parts of the plant, roots have also been found to release diverse volatiles that defend the plants from belowground insect pests by acting as antimicrobial and antiherbivore, and also by attracting the natural enemies of the root feeding insect pests.Citation7 Root feeding insect, Diuraphis noxia (Mord.) triggers the emission of 1, 8-cineole, a monoterpene volatile, which isis toxic and repellent to some insects.Citation110 Sesquiterpene (E)-β- caryophyllene produced by maize roots in response to feeding by the larvae of Diabrotica virgifera virgifera LeConte attracts the nematode H. megidi.Citation111 However, root emitted volatiles such as 1,8-Cineole inhibits the growth of Brassica campestris seedlings due to the inhibition cell proliferation more severely than cell elongation because root growth requires both elongation and proliferation of the constituent cells,Citation112 and also due to the interference with nuclear as well as organelle DNA synthesis in root apical meristem and alteration in root phospholipids and sterol composition.Citation113
Defense elicitors (insect oral secretion)
Plants undergo a dynamic change in transcriptomes, proteomes, and metabolomes in response to herbivore-induced physical and chemical cues such as insect oral secretions (OS) and compounds in the oviposition fluids. It is generally believed that insect-induced plant responses are mediated by oral secretions and regurgitates of the herbivore. The defenses generated by various elicitors differ based on the type of the elicitor and the biological processes involved.Citation114 A potential elicitor of herbivore-induced plant volatiles from the regurgitate of Pieris brassicae L. larvae has been identified as β-glucosidase which results in emission of a volatile blend from mechanically wounded cabbage leaves that attract the parasitic wasp, Cotesia glomerata (L.).Citation115
Fatty acid-amino acid conjugates (FACs) are the major components in the oral secretions of insects. The first FAC elicitor identified was volicition, N-(17-hydroxylinolenoyl)-L-glutamine (volicitin), detected in the OS of beet armyworm larvae, S. exigua.Citation116 Volicitin when applied on Zea mays L. induced the emission of elicitor that attracts the natural enemies of the feeding larvae.Citation116 N-linolenoyl-glu isolated from regurgitate of tobacco hornworm, M. sexta has been found to be a potential elicitor of volatile emissions in tobacco plants.Citation117 The FACs in OS of insects have been found to activate mitogen-activated protein kinase (MAPK) pathway, that regulate plant growth and development, and play an important role in signaling transduction in responses to various stresses including cold, heat, ROS, UV, drought, pathogen and insect attack.Citation118 FACs in oral secretions of M. sexta, when applied to the wounded leaves have been found to activate signaling processes that lead to the activation of MAPKs, salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK), and bursts of jasmonic acid (JA), JA-isoleucine conjugate (JA-Ile), salicylic acid (SA), and ethylene.Citation118,Citation119 In wild rice, Oryza minuta Presl., expression of putative MAPK, OmMKKI, is induced by brown plant hopper, N. lugens feeding.Citation120 Several other FAC elicitors such as N-acyl Gln/Glu have been isolated from regurgitates of various lepidopteran species.Citation8 The FACs has also been reported to induce accumulation of 7-epi-jasmonic acid, an octadecanoid-derived phytohormone, which is a potent elicitor of transcripts of herbivore-responsive genes in tobacco plants.Citation117 The FACs in lepidopteran OS evoke specific responses such as transcriptomic and proteomic alteration, induction of nicotine, and proteinase inhibitors in N. attenuate.Citation121 Besides FACs, other groups of elicitors identified in insect oral secretions include inceptins,Citation122 and caeliferins.Citation123 Inceptins are disulphide-bonded peptides formed by the proteolytic fragmentation of plastidic ATP synthase, γ-subunit,Citation122 whereas caeliferins are sulfated fatty acids, in the oral secretion of S. americana (Stal.), and other grasshopper species.Citation123 The lipase activity of grasshopper oral secretions evoked an immediate and quick accumulation of various oxylipins, such as, 13-hydroperoxy octadecatrienoic acid, 12-oxo-phytodienoic acid (OPDA), JA, and jasmonic acid-isoleucine in Arabidopsis.Citation124 Furthermore, there was increase in cytosolic calcium, ethylene emission and activity of MAPKs on treatment with grasshopper oral secretions.Citation124
Role of phytohormones in induced resistance in plants
Plant defense against herbivore attack involves many signal transduction pathways that are mediated by a network of phytohormones. Plant hormones play a critical role in regulating plant growth, development, and defense mechanisms.Citation2 A number of plant hormones have been implicated in intra- and inter-plant communication in plants damaged by herbivores. Most of the plant defense responses against insects are activated by signal-transduction pathways mediated by JA, SA, and ethylene.Citation79,Citation125 Specific sets of defense related genes are activated by these pathways upon wounding or by insect feeding. These hormones may act individually, synergistically or antagonistically, depending upon the attacker.
Jasmonic acid
Although various phytohormones are involved in plant defense against herbivores, JA is the most important phytohormone linked to plant defense against herbivores and activates the expression of both direct and indirect defenses.Citation4,Citation5,Citation125 JA is derived from linolenic acid through octadecanoid pathway and accumulates upon wounding and herbivory in plant tissues.Citation83 Chewing of plant parts by insects causes the dioxygenation of linoleic acid (18:2) and linolenic acid (18:3) by specific LOXs at C9 or C13 to form (9S)- or (13S)-hydroperoxy-octadecadi(tri)enoic acids, which are converted into 12-oxophytodienoic acid (12-OPDA) by allene oxide synthase and allene oxide cyclase. OPDA is transferred to the peroxisome, where it is reduced by OPDA reductase 3 (OPR3), forming JA. Oxidative burst produces ROS, which convert linolenic acid into phytoprostanes that signal transduction pathways.Citation93 A broad spectrum of defensive responses are induced by jasmonates that include antioxidative enzymes, PIs, VOCs, alkaloid production, trichome formation, and secretion of EFN.Citation88,Citation102,Citation114 A large numbers of genes involved in defense against herbivores are regulated by JA.Citation125 Concentration of indole glucosinolate, an important defensive compound, is induced by jasmonates In addition to its role in the production of JA, OPDA signals the defense pathways individually. For example, OPDA signaling regulates the CORONATIN-INSENSITIVE 1 (COI1) -dependent and -independent transcription,Citation126 alters the intracellular calcium levels and cellular redox status.Citation127 Jasmonates (most likely the JA-amino acid conjugate jasmonoyl–isoleucine) have been found to interact with the COI1 unit of an E3 ubiquitin ligase complex, termed SCFCOI1 (Skip/Cullin/F-box–COI1), which promotes binding of the COI1-unit to JAZ (jasmonate ZIM-domain) proteins, resulting in degradation of JAZ proteins, which otherwise suppress JA-inducible gene expression.Citation128 JA has also been reported to affect calcium-dependent protein kinases (CDPK) transcript, and activity in potato plants.Citation129 CDPKs comprise of a large family of serine/threonine kinases in plants (34 members in Arabidopsis) and play an important role in plant defense against a variety of biotic and abiotic stresses through signal transduction.Citation130 In addition to the role played by JA in direct resistance against insect pests through the induction of various defensive compounds, its role in indirect resistance has also been well established.Citation86 For example, EFN produced by JA is used as an alternate food by natural enemies of insect pests.Citation131 JA also induces the defense enzymes such as POD,Citation4,Citation5,Citation125 and PPO.Citation4,Citation5,Citation80
Salicylic acid
Salicylic acid (SA), a benzoic acid derivative, is an important phytohormone involved in regulation of plant defense.Citation132 It is an important endogenous plant growth regulator that generates a wide range of metabolic and physiological responses in plants involved in defense in addition to their impact on plant growth and development.Citation133 Responses to SA depend on a regulatory protein called Non-Expressor of Pathogenesis-Related Genes1 (NPR1).Citation134 The NPR1 gene is activated through redox pathways by SA accumulation and is translocated to the nucleus, however, it does not bind to DNA directly, but acts through transcription factors.Citation134 SA induces greater defense against piercing and sucking type of insect pests than the chewing ones.Citation80 SA signaling molecule is involved in local defense as well as induction of systemic resistance. Production of ROS by SA pathway has been proposed to induce resistance in plants against insect pests, e.g., in tomato plants against H. armigera.Citation135 H2O2 induced by SA in plants defends them against various insect pests since H2O2 actively damages the digestive system of insects leading to reduced growth and development.Citation35,Citation135 Furthermore, SA signals the release of plant volatiles that attract the natural enemies of insect pests, e.g., Lima bean and tomato plants infested by spider mite attract the natural enemies of spider mite.Citation97 However, it has been reported that SA and JA act antagonistically, where SA inhibits the activity of JA and vice versa.Citation35 MeSA serves as a volatile signal to trigger induced defenses in plants, including HIPV emission, and a number of predaceous arthropods are attracted to MeSA under field conditions.Citation35,Citation97
Ethylene
Ethylene is an important phytohormone, which plays an active role in plant defense against many insects.Citation136 Ethylene signaling pathway plays an important role in induced plant defense against herbivores and pathogens both directly and indirectly,Citation136 however, there are limited reports on its role in indirect defense through the emission of HIPVs.Citation137 ET signaling pathway works either synergistically or antagonistically,Citation138 with JA in expression of plant defense responses against pathogens and herbivorous insects. It has been reported that ET and JA work together in tomato in PIs expression.Citation138 Infestation by A. alni induced the emission of ethylene and release of various valatiles in Alnus glutinosa L. leaves in addition to mono-, sesqui and homoterpenes.Citation85 ET precursor, 1-aminocyclopropane-1-carboxylic acid has been reported to enhance the volatile emission from the JA treated detached leaves.Citation107 Ethylene further induced the emission of volatiles induced by volicitin, JA or (Z)-3-hexen-ol in maize.Citation105
Role of Calcium ions (Ca2+) in plant defense
Plant defense elicitors induced in plants upon herbivory undergo different signal transduction pathways. Ca2+ signaling is one of the early events in insect-plant interaction, where Ca2+ acts as a second messenger, which in turn mediates a number of plant signaling pathways.Citation35 Herbivore induced signals rapidly spread over the leaf and leads to a strong Ca2+-dependent transmembrane potential (Vm) depolarization in the damage zone, and is followed by a transient Vm hyperpolarization in the surrounding area, and a constant depolarization at distances greater than 6–7 mm.Citation35,Citation139 Organelle and apoplastic fluid Ca2+ concentration is generally higher (about 104 to 105 times) as compared with that in the cytosol (100 and 200 nM.). However, upon insect attack, the cytosolic Ca2+ increases, which in turn activates the calcium-sensing proteins such as calmodulin, calmodulin-binding proteins, and calcium-dependent protein kinases (CDPKs) that promote the signaling events such as, phosphorylation and transcriptional change.Citation139,Citation140 However, CDPKs are the important proteins against biotic and abiotic stresses, which formCa2+ sensors that contain a protein kinase domain and a calmodulin like domain (including an EF-hand calcium-binding site) in a single polypeptide.Citation130,Citation141 NtCDPK2 regulates the activation of stress-induced MAP kinases in tobacco.Citation142 Involvement of two Arabidopsis CPKs (CPK3 and CPK13) in herbivory-induced signaling network through HsfB2a-mediated regulation of the defense-related transcriptional machinery has been observed in tobacco.Citation139 Damage by S. littoralis larvae on Phaseolus lunatus L. induced Ca2+ not only in cells adjacent to the feeding site, but throughout the leaf.Citation35 Expression of calmodulin binding proteins involved in plant defense signaling increased considerably in wheat damaged by D. noxia and Arabidopsis by M. persicae.Citation140
Role of reactive oxygen species (ROS) in plant defense
Oxidative state of plants is an important tactic that enables plants to defend against various stresses. Rapid and transient generation of ROS is a common phenomenon in plants on account of oxidative stress due to biotic and abiotic factors.Citation35,Citation141 ROS play versatile signaling functions that mediate multiple responses, and can also act directly as toxins. However, production of ROS on account of biotic stress is still debatable.Citation35 ROS include partially reduced forms of oxygen such as superoxide (O-), hydrogen peroxide (H2O2), and hydroxyl radicals (HO-).Citation35,Citation141,Citation142 Distinct signaling pathways are activated by different types of ROS especially the ones involving MAPKs.Citation141,Citation142 Rapid increase in ROS content under stress conditions is referred as ‘oxidative burst.”Citation35 Following insect attack, ROS accumulate in apoplastic as well as in symplastic regions, besides their main concentration in exocellular matrix, peroxisomes/mitochondria, and plasma membrane.Citation35,Citation141 Apoplastic burst of ROS acts as a first barrier against subsequent attack by the pathogens and herbivores.Citation65 Being highly reactive, ROS can potentially react with and/or cause damage to proteins, lipids, and nucleic acids. However, to prevent the self-toxicity of ROS, plant cells have developed ROS scavenging systems for removing the excess ROS to maintain a relatively low and constant ROS concentration.Citation1,Citation35
Among all the ROS, high stability and freely diffusible H2O2 is a central component of induced defense response in plants against different stresses.Citation35,Citation141,Citation142 Although H2O2 is produced in various ways, the oxidative burst is supposed to occur through the activation of membrane bound NADPH complex. NADPH oxidase generates superoxide anion at the plasma membrane or in the apoplast extracellularly, which is then converted to H2O2 by superoxide dismutase (SOD).Citation35,Citation141 Besides having direct effect on the pathogens and herbivores, H2O2 stimulates a cascade of reactions that lead to the expression of defense genes, which prevent the plants from subsequent attack by pathogens and herbivores.Citation141 H2O2 application in Arabidopsis results in up- and downregulation of many genes (113 and 62 genes, respectively), suggesting that ROS act as secondary messengers to control gene expression.Citation143 ROS also play an important role in mediating cross-linking of cell wall components by peroxidase, and also for the activation of many defenses related genes.Citation141 Oxidative changes in plants after insect attack cause oxidative damage to insect midgut, mainly due to accumulation of H2O2.Citation35,Citation141 Many physiological and molecular responses in plants against insect attack are triggered by H2O2, and its levels remain elevated as long as the herbivore attack persists.Citation35,Citation141 Induction of H2O2 has been studied in oat, wheat, barley and groundnut against D. noxia, R. padi, Schizaphis graminum Rond., H. armigera and S. litura.Citation4,Citation5,Citation132,Citation139 Argandona et al.Citation144 observed induction of H2O2 in barley infested with S. graminum after 20 min of infestation, indicating that H2O2 could be the beginning of a cascade of physiological and molecular events leading to production of further defensive components, and protection of plants from subsequent damage. ROS mediate the defense gene activation and establish additional defenses by regulating the transcription and/or by interacting with other signal components like phosphorylation in plant systems in response to a variety of stresses.Citation35,Citation141
Gene expression: The basic process of plant defense
Extensive rearrangements in gene expression occur in plants in response to herbivory with hundreds, and even up to several thousands of genes getting up- or downregulated.Citation57,Citation145 Advances in genomics and transcriptomics including availability of whole-genome sequence data, expressed sequence tags (ESTs), and microarrays, has led to better understanding of the changes in gene-expression profiles in response to insect attack.Citation139,Citation146,Citation147
DNA microarrays provide a closer and complete view of gene-expression patterns and signaling responses mediated by insect elicitors and plant signals, and has proven to be exceptional tools to monitor the expression of thousands of genes simultaneously.Citation147 However, with the advent of next-generation sequencing (NGS) technologies, it is anticipated that microarrays will be soon replaced by some new and innovative technologies like RNA-sequencing, RAD-sequencing, and reduced represented sequencing etc., for measuring gene expression directly. Expression quantitative trait loci (eQTL) mapping has revolutionized the area of gene expression. The eQTL mapping is having the advantage of dealing with thousands of traits at a time and has been used in many plants including Arabidopsis and rice.Citation148 Investigation of inducible defenses in Arabidopsis against P. rapae and Brassica oleracea var capitata L. and Brassica nigra L., against caterpillar P. rapae or the aphid Brevicoryne brassicae L. by microarrays has been studied extensively.Citation147,Citation149 Responses against feeding of Diuraphis noxia (Mord.), S. graminum, M. nicotianae, M. persicae and S. avenae on foliage of Arabidopsis, celery, sorghum, Apium graveolens L. cereal, tobacco or wheat plants have been well established.Citation80,Citation92,Citation150
Change in gene expression profiles after herbivory has shown a substantial reallocation of plant resources to defense. Gene expression levels have also been used to analyze the differences in transcriptional profiles of different genotypes within a plant species.Citation89,Citation147 A large numbers of genes (2182) are expressed by the aphid, M. persicae as compared with caterpillar, P. rapae (186) attack.Citation149 Lepidopterans usually elicit changes in the expression of genes involved in glucosinolate metabolism in Brassicaceae, detoxification, cell survival, and signal transduction,Citation149 while the aphids regulate the expression of genes involved in cell wall modifications, oxidative stress, calcium-dependent signaling, and glucosinolate synthesis.Citation146 Different attackers face different responses in plants based on the feeding behavior and the plant attacked; e.g., transcriptional changes in Arabidopsis thaliana in response to feeding by aphid, M. persicae and whitefy, Bemisia tabaci (Gen.).Citation151,Citation152 Different plants respond differently to the same herbivore, e.g., two white cabbage cultivars differ considerably in gene expression in response to feeding by P. rapae.Citation147 Combination of various technologies such as genetic, genomic tools including microarrays, deep sequencing, and transcriptional profiling tools and proteomics through mass spectrometry will advance our understanding of molecular mechanisms of plant defense against insect herbivores to a greater extent.
Transgenerational induced resistance to herbivores
Biotic and abiotic stresses in plants have been found to induce resistance not only in the maternal plants, but also in the offsprings.Citation153 This maternally induced resistance (transgenerational immunity) has been found to protect the progeny of plants exposed to herbivory from insect pests, besides producing vigorous seeds and seedlings.Citation153 However, there are a few reports on transgenerational immunity of plants against insect pests. Wild radish plants, Raphanus raphanistrum damaged by P. rapae or treated with JA produce offspring’s with high levels of induced resistance to this insect.Citation154 Arabidopsis plants exposed to stresses such as, cold, heat and flood, resulted in a higher homologous recombination frequency and increased genome methylation, which in turn induced the resistance to stress in the progeny.Citation155 Maternal plants with low to intermediate levels of herbivore damage could produce the seeds that are more vigorous and seedlings that are resistant to insect pests.154However, further studies are required to understand the genetic and molecular mechanisms of such signaling interactions. Furthermore, research on plant-insect interactions should be focused not only to genetic effects, but also toward the epigenetic regulation of plant defense pathways and insect responses, because a substantial body of evidence has been demonstrated for mobile siRNA signals and inheritance of DNA methylation based changes in gene expression changes. There is much need for in-depth studies on this subject to exploit it for pest management by manipulating the maternal ecology. An understanding of transgenerational induced resistance might answer some of the intricate questions regarding the ability of plants to withstand herbivore damage.
Future Outlook
Although induced resistance has attained a considerable momentum recently, and has attracted the attention of scientists in evolutionary ecology, entomology, plant physiology, and biotechnology, much of the underlying mechanism have still remained unanswered. There is a need to understand the herbivore-specific signal molecules, their identification, mode of action, and further signal transduction. Since a single attribute can affect the herbivores and/or natural enemies positively and/or negatively, understanding of the multitrophic interactions is important to know the consequences of supposed defensive traits of a plant for use in pest management. An understanding of induced resistance in plants can be utilized for interpreting the ecological interactions between plants and herbivores and for exploiting in pest management in crops. Since the biochemical pathways that lead to induced resistance are highly conserved among the plants, the elicitors of these pathways could be used as inducers in many crops. The future challenge is to exploit the elicitors of induced defense in plants for pest management, and identify the genes encoding proteins that are up and/or downregulated during plant response to the herbivore attack, which can be deployed for conferring resistance to the herbivores through genetic transformation. However, before using an elicitor effectively in agricultural systems, it is important to understand the chemical changes they induce in the plant, the effect of these chemicals on the herbivores especially in the field, and to see if there is any alteration in plant growth and yield. The Eco-genomic approach which includes association and correlation studies, natural selection mapping, and population genomics enables the estimation of variable selection at (sets of) loci, and differentiates this from processes acting on the whole genome, such as migration and genetic drift. Eco-genomics needs to be much more explored and the consequences of (plasticity in) expression of genes for community processes need to be well understood, since the shape of a particular interspecific interaction is ubiquitous. Moreover, this approach enables the linking of different sub cellular processes to particular community structures, and the big challenge ahead is the implementation of these results in a spatial framework.
References
- Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol 2008; 59:41 - 66; http://dx.doi.org/10.1146/annurev.arplant.59.032607.092825; PMID: 18031220
- Verhage A, van Wees SCM, Pieterse CMJ. Plant immunity: it’s the hormones talking, but what do they say?. Plant Physiol 2010; 154:536 - 40; http://dx.doi.org/10.1104/pp.110.161570; PMID: 20921180
- Hare JD. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 2011; 56:161 - 80; http://dx.doi.org/10.1146/annurev-ento-120709-144753; PMID: 21133760
- Usha Rani P, Jyothsna Y. Biochemical and enzymatic changes in rice as a mechanism of defense. Acta Physiol Plant 2010; 32:695 - 701; http://dx.doi.org/10.1007/s11738-009-0449-2
- War AR, Paulraj MG, War MY, Ignacimuthu S. Jasmonic acid- mediated induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J Plant Growth Regul 2011; a 30:512 - 23; http://dx.doi.org/10.1007/s00344-011-9213-0
- War AR, Paulraj MG, War MY, Ignacimuthu S. Herbivore- and elicitor-induced resistance in groundnut to Asian armyworm, Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Plant Signal Behav 2011; b 6:1769 - 77; http://dx.doi.org/10.4161/psb.6.11.17323; PMID: 22042128
- Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 2006; 25:417 - 40; http://dx.doi.org/10.1080/07352680600899973
- Arimura GI, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 2009; 50:911 - 23; http://dx.doi.org/10.1093/pcp/pcp030; PMID: 19246460
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in antiherbivore defense. Perspec. Plant Ecol Evol Syst 2007; 8:157 - 78; http://dx.doi.org/10.1016/j.ppees.2007.01.001
- Karban R. The ecology and evolution of induced resistance against herbivores. Funct Ecol 2011; 25:339 - 47; http://dx.doi.org/10.1111/j.1365-2435.2010.01789.x
- Sharma HC. Biotechnological Approaches for Pest Management and Ecological Sustainability. CRC Press/Taylor and Francis, New York, USA 2009; pp. 526.
- Agrawal AA. Current trends in the evolutionary ecology of plant defence. Funct Ecol 2011; 25:420 - 32; http://dx.doi.org/10.1111/j.1365-2435.2010.01796.x
- Agrawal AA, Janssen A, Bruin J, Posthumus MA, Sabelis MW. An ecological cost of plant defence: attractiveness of bitter cucumber plants to natural enemies of herbivores. Ecol Lett 2002; 5:377 - 85; http://dx.doi.org/10.1046/j.1461-0248.2002.00325.x
- Miranda M, Ralph SG, Mellway R, White R, Heath MC, Bohlmann J, et al. The transcriptional response of hybrid poplar (Populus trichocarpa x P. deltoides) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol Plant Microbe Interact 2007; 20:816 - 31; http://dx.doi.org/10.1094/MPMI-20-7-0816; PMID: 17601169
- Steppuhn A, Baldwin IT. Resistance management in a native plant: nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol Lett 2007; 10:499 - 511; http://dx.doi.org/10.1111/j.1461-0248.2007.01045.x; PMID: 17498149
- Agrawal AA, Fishbein M, Jetter R, Salminen JP, Goldstein JB, Freitag AE, et al. Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): chemistry, ecophysiology, and insect behavior. New Phytol 2009; 183:848 - 67; http://dx.doi.org/10.1111/j.1469-8137.2009.02897.x; PMID: 19522840
- Duffey SS, Stout MJ. Antinutritive and toxic components of plant defense against insects. Arch Insect Biochem Physiol 1996; 32:3 - 37; http://dx.doi.org/10.1002/(SICI)1520-6327(1996)32:1<3::AID-ARCH2>3.0.CO;2-1
- Sharma HC, Sujana G, Rao DM. Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeonpea. Arthropod-Plant Interact 2009; 3:151 - 61; http://dx.doi.org/10.1007/s11829-009-9068-5
- He J, Chen F, Chen S, Lv G, Deng Y, Fang W, et al. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J Plant Physiol 2011; 168:687 - 93; http://dx.doi.org/10.1016/j.jplph.2010.10.009; PMID: 21145126
- Chamarthi SK, Sharma HC, Sahrawat KL, Narasu LM, Dhillon MK. Physico-chemical mechanisms of resistance to shoot fly, Atherigona soccata in sorghum, Sorghum bicolor.. J Appl Entomol 2010; 135:446 - 55; http://dx.doi.org/10.1111/j.1439-0418.2010.01564.x
- Handley R, Ekbom B, Agren J. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana.. Ecol Entomol 2005; 30:284 - 92; http://dx.doi.org/10.1111/j.0307-6946.2005.00699.x
- Agrawal AA. Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 1999; 80:1713 - 23; http://dx.doi.org/10.1890/0012-9658(1999)080[1713:IRTHIW]2.0.CO;2
- Dalin P, Björkman C. Adult beetle grazing induces willow trichome defence against subsequent larval feeding. Oecologia 2003; 134:112 - 8; http://dx.doi.org/10.1007/s00442-002-1093-3; PMID: 12647188
- Bjorkman C, Ahrne K. Influence of leaf trichome density on the efficiency of two polyphagous insect predators. Entomol Exp Appl 2005; 115:179 - 86; http://dx.doi.org/10.1111/j.1570-7458.2005.00284.x
- Traw MB. Is induction response negatively correlated with constitutive resistance in black mustard?. Evolution 2002; 56:2196 - 205; PMID: 12487350
- Baur R, Binder S, Benz G. Nonglandular leaf trichomes as short-term inducible defense of the grey alder, Alnus incana (L), against the chrysomelid beetle, Agelastica alni L. Oecologia 1991; 87:219 - 26; http://dx.doi.org/10.1007/BF00325259
- Olson DL, Nechols JR. Effects of squash leaf trichome exudates and honey on adult feeding, survival, and fecundity of the squash bug (Heteroptera, Coreidae) egg parasitoid Gryon pennsylvanicum (Hymenoptera: Scelionidae). Environ Entomol 1995; 24:454 - 8
- Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, et al. beta-Glucosidases as detonators of plant chemical defense. Phytochemistry 2008; 69:1795 - 813; http://dx.doi.org/10.1016/j.phytochem.2008.03.006; PMID: 18472115
- Walling LL. The myriad plant responses to herbivores. J Plant Growth Regul 2000; 19:195 - 216; PMID: 11038228
- Nuessly GS, Scully BT, Hentz MG, Beiriger R, Snook ME, Widstrom NW. Resistance to Spodoptera frugiperda (Lepidoptera: Noctuidae) and Euxesta stigmatias (Diptera: Ulidiidae) in sweet corn derived from exogenous and endogenous genetic systems. J Econ Entomol 2007; 100:1887 - 95; http://dx.doi.org/10.1603/0022-0493(2007)100[1887:RTSFLN]2.0.CO;2; PMID: 18232407
- Chamarthi SK, Sharma HC, Vijay PM, Narasu LM. Leaf surface chemistry of sorghum seedlings influencing expression of resistance to sorghum shoot fly, Atherigona soccata.. J Plant Biochem Biotechnol 2011; 20:211 - 6; http://dx.doi.org/10.1007/s13562-011-0048-3
- Barakat A, Bagniewska-Zadworna A, Frost CJ, Carlson JE. Phylogeny and expression profiling of CAD and CAD-like genes in hybrid Populus (P. deltoides x P. nigra): evidence from herbivore damage for subfunctionalization and functional divergence. BMC Plant Biol 2010; 10:100; http://dx.doi.org/10.1186/1471-2229-10-100; PMID: 20509918
- Johnson MTJ, Smith SD, Rausher MD. Plant sex and the evolution of plant defenses against herbivores. Proc Natl Acad Sci U S A 2009; 106:18079 - 84; http://dx.doi.org/10.1073/pnas.0904695106; PMID: 19617572
- Bhonwong A, Stout MJ, Attajarusit J, Tantasawat P. Defensive role of tomato polyphenol oxidases against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). J Chem Ecol 2009; 35:28 - 38; http://dx.doi.org/10.1007/s10886-008-9571-7; PMID: 19050959
- Maffei ME, Mithöfer A, Boland W. Insects feeding on plants: rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 2007; 68:2946 - 59; http://dx.doi.org/10.1016/j.phytochem.2007.07.016; PMID: 17825328
- Simmonds MSJ. Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry 2003; 64:21 - 30; http://dx.doi.org/10.1016/S0031-9422(03)00293-0; PMID: 12946403
- Treutter D. Significance of flavonoids in plant resistance: a review. Environ Chem Lett 2006; 4:147 - 57; http://dx.doi.org/10.1007/s10311-006-0068-8
- Simmonds MSJ, Blaney WM, Fellows LE. Behavioural and electrophysiological study of antifeedant mechanisms associated with polyhydroxyalkaloids. J Chem Ecol 1990; 16:3167 - 96; http://dx.doi.org/10.1007/BF00979618
- Johnson ET, Dowd PF. Differentially enhanced insect resistance, at a cost, in Arabidopsis thaliana constitutively expressing a transcription factor of defensive metabolites. J Agric Food Chem 2004; 52:5135 - 8; http://dx.doi.org/10.1021/jf0308049; PMID: 15291486
- Lane GA, Sutherland ORW, Skipp RA. Isoflavonoids as insect feeding deterrents and antifungal components from root of Lupinus angustifolius.. J Chem Ecol 1987; 13:771 - 83; http://dx.doi.org/10.1007/BF01020159
- Simmonds MSJ, Stevenson PC. Effects of isoflavonoids from Cicer on larvae of Heliocoverpa armigera.. J Chem Ecol 2001; 27:965 - 77; http://dx.doi.org/10.1023/A:1010339104206; PMID: 11471948
- Renwick JAA, Zhang W, Haribal M, Attygalle AB, Lopez KD. Dual chemical barriers protect a plant against different larval stages of an insect. J Chem Ecol 2001; 27:1575 - 83; http://dx.doi.org/10.1023/A:1010402107427; PMID: 11521397
- Sharma HC, Agarwal RA. Role of some chemical components and leaf hairs in varietal resistance in cotton to jassid, Amrasca biguttula biguttula Ishida. J Entomol Res 1983; 7:145 - 9
- Barbehenn RV, Peter Constabel C. Tannins in plant-herbivore interactions. Phytochemistry 2011; 72:1551 - 65; http://dx.doi.org/10.1016/j.phytochem.2011.01.040; PMID: 21354580
- Peters DJ, Constabel CP. Molecular analysis of herbivore-induced condensed tannin synthesis: cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J 2002; 32:701 - 12; http://dx.doi.org/10.1046/j.1365-313X.2002.01458.x; PMID: 12472686
- Roitto M, Rautio P, Markkola A, Julkunen-Tiitto R, Varama M, Saravesi K, et al. Induced accumulation of phenolics and sawfly performance in Scots pine in response to previous defoliation. Tree Physiol 2009; 29:207 - 16; http://dx.doi.org/10.1093/treephys/tpn017; PMID: 19203946
- Hikosaka K, Takashima T, Kabeya D, Hirose T, Kamata N. Biomass allocation and leaf chemical defence in defoliated seedlings of Quercus serrata with respect to carbon-nitrogen balance. Ann Bot 2005; 95:1025 - 32; http://dx.doi.org/10.1093/aob/mci111; PMID: 15760913
- Keinanen M, Julkunen-Tiitto R, Mutikainen P, Walls M, Ovaska J, Vapaavuori E. Trade-offs in phenolic metabolism of silver birch: effects of fertilization, defoliation, and genotype. Ecology 1999; 80:1970 - 86
- Feeny PP. Effect of oak leaf tannins on larval growth of the winter moth Operophtera brumata.. J Insect Physiol 1968; 14:805 - 17; http://dx.doi.org/10.1016/0022-1910(68)90191-1
- Bernays EA. Plant tannins and insect herbivores: an appraisal. Ecol Entomol 1981; 6:353 - 60; http://dx.doi.org/10.1111/j.1365-2311.1981.tb00625.x
- Grayer RJ, Kimmins FM, Padgham DE, Harborne JB, Ranga Rao DV. Condensed tannin levels and resistance in groundnuts (Arachis hypogoea (L.)) against Aphis craccivora (Koch). Phytochemistry 1992; 31:3795 - 9; http://dx.doi.org/10.1016/S0031-9422(00)97530-7
- Bryant JP, Reichardt PB, Clausen TP, Werner RA. Effects of mineral nutrition on delayed induced resistance in Alaska paper birch. Ecology 1993; 74:2072 - 84; http://dx.doi.org/10.2307/1940853
- Mellway RD, Tran LT, Prouse MB, Campbell MM, Constabel CP. The wound-, pathogen-, and ultraviolet B-responsive MYB134 gene encodes an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in poplar. Plant Physiol 2009; 150:924 - 41; http://dx.doi.org/10.1104/pp.109.139071; PMID: 19395405
- Bernays EA, Chapman RF. Plant secondary compounds and grasshoppers: beyond plant defenses. J Chem Ecol 2000; 26:1773 - 93; http://dx.doi.org/10.1023/A:1005578804865
- Gulsen O, Eickhoff T, Heng-Moss T, Shearman R, Baxendale F, Sarath G, et al. Characterization of peroxidase changes in resistant and susceptible warm-season turfgrasses challenged by Blissus occiduus.. Arthropod-Plant Interact 2010; 4:45 - 55; http://dx.doi.org/10.1007/s11829-010-9086-3
- Chen H, Wilkerson CG, Kuchar JA, Phinney BS, Howe GA. Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc Natl Acad Sci U S A 2005; 102:19237 - 42; http://dx.doi.org/10.1073/pnas.0509026102; PMID: 16357201
- Chen Y, Ni X, Buntin GD. Physiological, nutritional, and biochemical bases of corn resistance to foliage-feeding fall armyworm. J Chem Ecol 2009; 35:297 - 306; http://dx.doi.org/10.1007/s10886-009-9600-1; PMID: 19221843
- Chakraborti D, Sarkar A, Mondal HA, Das S. Tissue specific expression of potent insecticidal, Allium sativum leaf agglutinin (ASAL) in important pulse crop, chickpea (Cicer arietinum L.) to resist the phloem feeding Aphis craccivora.. Transgenic Res 2009; 18:529 - 44; http://dx.doi.org/10.1007/s11248-009-9242-7; PMID: 19184504
- Vandenborre G, Smagghe G, Van Damme EJM. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 2011; 72:1538 - 50; http://dx.doi.org/10.1016/j.phytochem.2011.02.024; PMID: 21429537
- Saha P, Majumder P, Dutta I, Ray T, Roy SC, Das S. Transgenic rice expressing Allium sativum leaf lectin with enhanced resistance against sap-sucking insect pests. Planta 2006; 223:1329 - 43; http://dx.doi.org/10.1007/s00425-005-0182-z; PMID: 16404581
- Macedo ML, Freire MDGM, Da Silva MB, Coelho LC. Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculates (Coleoptera: Bruchidae). Comp Biochem Physiol Mol Integr Physiol 2007; 146:486 - 98; http://dx.doi.org/10.1016/j.cbpa.2006.01.020
- Stoger E, William S, Christou P, Down RE, Gatehouse JA. Expression of the insecticidal lectin from snowdrop (Galanthus nivalis agglutinin; GNA) in transgenic wheat plants: effects on predation by the grain aphid Sitobion avenae.. Mol Breed 1999; 5:65 - 73; http://dx.doi.org/10.1023/A:1009616413886
- Dutta I, Saha P, Majumder P, Sarkar A, Chakraborti D, Banerjee S, et al. The efficacy of a novel insecticidal protein, Allium sativum leaf lectin (ASAL), against homopteran insects monitored in transgenic tobacco. Plant Biotechnol J 2005; 3:601 - 11; http://dx.doi.org/10.1111/j.1467-7652.2005.00151.x; PMID: 17147631
- Majumder P, Mondal HA, Das S. Insecticidal activity of Arum maculatum tuber lectin and its binding to the glycosylated insect gut receptors. J Agric Food Chem 2005; 53:6725 - 9; http://dx.doi.org/10.1021/jf051155z; PMID: 16104791
- Powell G, Tosh CR, Hardie J. Host plant selection by aphids: behavioral, evolutionary, and applied perspectives. Annu Rev Entomol 2006; 51:309 - 30; http://dx.doi.org/10.1146/annurev.ento.51.110104.151107; PMID: 16332214
- Yuan Z, Zhao C, Zhou Y, Tian YC. Aphid resistant transgenic tobacco plants expressing modified gna gene. Acta Bot Sin 2001; 43:592 - 7
- Sun X, Wu A, Tang K. Transgenic rice lines with enhanced resistance to the small brown planthopper. Crop Prot 2002; 21:511 - 4; http://dx.doi.org/10.1016/S0261-2194(01)00127-2
- Lannoo N, Vandenborre G, Miersch O, Smagghe G, Wasternack C, Peumans WJ, et al. The jasmonate-induced expression of the Nicotiana tabacum leaf lectin. Plant Cell Physiol 2007; 48:1207 - 18; http://dx.doi.org/10.1093/pcp/pcm090; PMID: 17623741
- Vandenborre G, Miersch O, Hause B, Smagghe G, Wasternack C, Van Damme EJM. Spodoptera littoralis-induced lectin expression in tobacco. Plant Cell Physiol 2009; 50:1142 - 55; http://dx.doi.org/10.1093/pcp/pcp065; PMID: 19416954
- Giovanini MP, Saltzmann KD, Puthoff DP, Gonzalo M, Ohm HW, Williams CE. A novel wheat gene encoding a putative chitin-binding lectin is associated with resistance against Hessian fly. Mol Plant Pathol 2007; 8:69 - 82; http://dx.doi.org/10.1111/j.1364-3703.2006.00371.x; PMID: 20507479
- Subramanyam S, Smith DF, Clemens JC, Webb MA, Sardesai N, Williams CE. Functional characterization of HFR1, a high-mannose N-glycan-specific wheat lectin induced by Hessian fly larvae. Plant Physiol 2008; 147:1412 - 26; http://dx.doi.org/10.1104/pp.108.116145; PMID: 18467454
- Puthoff DP, Sardesai N, Subramanyam S, Nemacheck JA, Williams CE. Hfr-2, a wheat cytolytic toxin-like gene, is up-regulated by virulent Hessian fly larval feeding. Mol Plant Biol 2005; 6:411 - 23
- Jiang JF, Han Y, Xing LJ, Xu YY, Xu ZH, Chong K. Cloning and expression of a novel cDNA encoding a mannose-specific jacalin-related lectin from Oryza sativa.. Toxicon 2006; 47:133 - 9; http://dx.doi.org/10.1016/j.toxicon.2005.10.010; PMID: 16359716
- Lawrence PK, Koundal KR. Plant protease inhibitors in control of phytophagous insects. Electron J Biotechnol 2002; 5:93 - 109; http://dx.doi.org/10.2225/vol5-issue1-fulltext-3
- Dunse KM, Stevens JA, Lay FT, Gaspar YM, Heath RL, Anderson MA. Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc Natl Acad Sci U S A 2010; 107:15011 - 5; http://dx.doi.org/10.1073/pnas.1009241107; PMID: 20696895
- Azzouz H, Cherqui A, Campan EDM, Rahbé Y, Duport G, Jouanin L, et al. Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphelinidae). J Insect Physiol 2005; 51:75 - 86; http://dx.doi.org/10.1016/j.jinsphys.2004.11.010; PMID: 15686649
- Hartl M, Giri AP, Kaur H, Baldwin IT. Serine protease inhibitors specifically defend Solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 2010; 22:4158 - 75; http://dx.doi.org/10.1105/tpc.109.073395; PMID: 21177479
- Zhu-Salzman K, Luthe DS, Felton GW. Arthropod-inducible proteins: broad spectrum defenses against multiple herbivores. Plant Physiol 2008; 146:852 - 8; http://dx.doi.org/10.1104/pp.107.112177; PMID: 18316640
- Gill RS, Gupta K, Taggar GK, Taggar MS. Role of oxidative enzymes in plant defenses against herbivory. Acta Phytopathol Entomol Hung 2010; 45:277 - 90; http://dx.doi.org/10.1556/APhyt.45.2010.2.4
- Zhao LY, Chen JL, Cheng DF, Sun JR, Liu Y, Tian Z. Biochemical and molecular characterizations of Sitobion avenae– induced wheat defense responses. Crop Prot 2009; 28:435 - 42; http://dx.doi.org/10.1016/j.cropro.2009.01.005
- Heng-Moss TM, Sarath G, Baxendale F, Novak D, Bose S, Ni X, et al. Characterization of oxidative enzyme changes in buffalograsses challenged by Blissus occiduus.. J Econ Entomol 2004; 97:1086 - 95; http://dx.doi.org/10.1603/0022-0493(2004)097[1086:COOECI]2.0.CO;2; PMID: 15279295
- Sethi A, McAuslane HJ, Rathinasabapathi B, Nuessly GS, Nagata RT. Enzyme induction as a possible mechanism for latex-mediated insect resistance in romaine lettuce. J Chem Ecol 2009; 35:190 - 200; http://dx.doi.org/10.1007/s10886-009-9596-6; PMID: 19184224
- Zhang SZ, Hau BZ, Zhang F. Induction of the activities of antioxidative enzymes and the levels of malondialdehyde in cucumber seedlings as a consequence of Bemisia tabaci (Hemiptera: Aleyrodidae) infestation. Arthropod-Plant Interact 2008; 2:209 - 13; http://dx.doi.org/10.1007/s11829-008-9044-5
- Stout MJ, Riggio MR, Yang Y, MJ. Direct induced resistance in Oryza sativa to Spodoptera frugiperda. Environ Entomol 2009; 38:1174 - 81; http://dx.doi.org/10.1603/022.038.0426; PMID: 19689897
- Tscharntke T, Thiessen S, Dolch R, Boland W. Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa.. Biochem Syst Ecol 2001; 29:1025 - 47; http://dx.doi.org/10.1016/S0305-1978(01)00048-5
- Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen JP. Hydrolyzable tannins as “quantitative defenses”: limited impact against Lymantria dispar caterpillars on hybrid poplar. J Insect Physiol 2009; 55:297 - 304; http://dx.doi.org/10.1016/j.jinsphys.2008.12.001; PMID: 19111746
- Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, van Loon JJA, Dicke M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: effects of time and dose, and comparison with induction by herbivores. J Exp Bot 2009; 60:2575 - 87; http://dx.doi.org/10.1093/jxb/erp101; PMID: 19451186
- Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, et al. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 2007; 25:1307 - 13; http://dx.doi.org/10.1038/nbt1352; PMID: 17982444
- Wang R, Shen WB, Liu LL, Jiang L, Liu YQ, Su N, et al. A novel lipoxygenase gene from developing rice seeds confers dual position specificity and responds to wounding and insect attack. Plant Mol Biol 2008; 66:401 - 14; http://dx.doi.org/10.1007/s11103-007-9278-0; PMID: 18185911
- Hildebrand DF, Rodriguez JG, Brown GC, Luu KT, Volden CS. Peroxidative responses of leaves in two soybean genotypes injured by twospotted spider mites (Acari: Tetranychidae). J Econ Entomol 1986; 79:1459 - 65
- Fidantsef AL, Stout MJ, Thaler JS, Duffey SS, Bostock RM. Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogenesisrelated protein P4 in the tomato, Lycopersicon esculentum.. Physiol Mol Plant Pathol 1999; 54:97 - 114; http://dx.doi.org/10.1006/pmpp.1998.0192
- Voelckel C, Weisser WW, Baldwin IT. An analysis of plant-aphid interactions by different microarray hybridization strategies. Mol Ecol 2004; 13:3187 - 95; http://dx.doi.org/10.1111/j.1365-294X.2004.02297.x; PMID: 15367131
- Rayapuram C, Baldwin IT. Increased SA in NPR1-silenced plants antagonizes JA and JA-dependent direct and indirect defenses in herbivore-attacked Nicotiana attenuata in nature. Plant J 2007; 52:700 - 15; http://dx.doi.org/10.1111/j.1365-313X.2007.03267.x; PMID: 17850230
- Maffei ME. Site of synthesis, biochemistry and functional role of plant volatiles. S Afr J Bot 2010; 76:612 - 31; http://dx.doi.org/10.1016/j.sajb.2010.03.003
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A 2004; 101:1781 - 5; http://dx.doi.org/10.1073/pnas.0308037100; PMID: 14749516
- Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 2006; 9:274 - 80; http://dx.doi.org/10.1016/j.pbi.2006.03.002; PMID: 16595187
- de Boer JG, Posthumus MA, Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol 2004; 30:2215 - 30; http://dx.doi.org/10.1023/B:JOEC.0000048784.79031.5e; PMID: 15672666
- Chen F, Tholl D, D’Auria JC, Farooq A, Pichersky E, Gershenzon J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 2003; 15:481 - 94; http://dx.doi.org/10.1105/tpc.007989; PMID: 12566586
- James DG. Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: methyl salicylate and the green lacewing, Chrysopa nigricornis. J Chem Ecol 2003; 29:1601 - 9; http://dx.doi.org/10.1023/A:1024270713493; PMID: 12921438
- Ulland S, Ian E, Mozuraitis R, Borg-Karlson AK, Meadow R, Mustaparta H. Methyl salicylate, identified as primary odorant of a specific receptor neuron type, inhibits oviposition by the moth Mamestra brassicae L. (Lepidoptera, noctuidae). Chem Senses 2008; 33:35 - 46; http://dx.doi.org/10.1093/chemse/bjm061; PMID: 17846100
- Yuan JS, Köllner TG, Wiggins G, Grant J, Degenhardt J, Chen F. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J 2008; 55:491 - 503; http://dx.doi.org/10.1111/j.1365-313X.2008.03524.x; PMID: 18433439
- Dickens JC. Plant volatiles moderate response to aggregation to pheromone in Colorado potato beetle. J Appl Entomol 2006; 130:26 - 31; http://dx.doi.org/10.1111/j.1439-0418.2005.01014.x
- Arimura G, Köpke S, Kunert M, Volpe V, David A, Brand P, et al. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol 2008; 146:965 - 73; http://dx.doi.org/10.1104/pp.107.111088; PMID: 18165324
- Arimura G, Ozawa R, Kugimiya S, Takabayashi J, Bohlmann J. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-beta-ocimene and transcript accumulation of (E)-beta-ocimene synthase in Lotus japonicus.. Plant Physiol 2004; 135:1976 - 83; http://dx.doi.org/10.1104/pp.104.042929; PMID: 15310830
- Ruther J, Kleier S. Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. J Chem Ecol 2005; 31:2217 - 22; http://dx.doi.org/10.1007/s10886-005-6413-8; PMID: 16132223
- Kessler A, Halitschke R, Diezel C, Baldwin IT. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata.. Oecologia 2006; 148:280 - 92; http://dx.doi.org/10.1007/s00442-006-0365-8; PMID: 16463175
- Horiuchi JI, Arimura GI, Ozawa R, Shimoda T, Dicke M, Takabayashi J, et al. Lima bean leaves exposed to herbivore-induced conspecific plant volatiles attract herbivores in addition to carnivores. Appl Entomol Zool (Jpn) 2003; 38:365 - 8; http://dx.doi.org/10.1303/aez.2003.365
- Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LL, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis.. Science 2005; 309:2070 - 2; http://dx.doi.org/10.1126/science.1116232; PMID: 16179482
- Schnee C, Köllner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci U S A 2006; 103:1129 - 34; http://dx.doi.org/10.1073/pnas.0508027103; PMID: 16418295
- Ro DK, Ehlting J, Keeling CI, Lin R, Mattheus N, Bohlmann J. Microarray expression profiling and functional characterization of AtTPS genes: duplicated Arabidopsis thaliana sesquiterpene synthase genes At4g13280 and At4g13300 encode root-specific and wound-inducible (Z)-gamma-bisabolene synthases. Arch Biochem Biophys 2006; 448:104 - 16; http://dx.doi.org/10.1016/j.abb.2005.09.019; PMID: 16297850
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005; 434:732 - 7; http://dx.doi.org/10.1038/nature03451; PMID: 15815622
- Yoshimura H, Sawai Y, Tamotsu S, Sakai A. 1,8-cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J Chem Ecol 2011; 37:320 - 8; http://dx.doi.org/10.1007/s10886-011-9919-2; PMID: 21344180
- Nishida N, Tamotsu S, Nagata N, Saito C, Sakai A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J Chem Ecol 2005; 31:1187 - 203; http://dx.doi.org/10.1007/s10886-005-4256-y; PMID: 16124241
- Pauwels L, Inzé D, Goossens A. Jasmonate-inducible gene: What does it mean?. Trends Plant Sci 2009; 14:87 - 91; http://dx.doi.org/10.1016/j.tplants.2008.11.005; PMID: 19162528
- Mattiacci L, Dicke M, Posthumus MA. beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci U S A 1995; 92:2036 - 40; http://dx.doi.org/10.1073/pnas.92.6.2036; PMID: 11607516
- Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997; 276:945 - 9; http://dx.doi.org/10.1126/science.276.5314.945
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 2001; 125:711 - 7; http://dx.doi.org/10.1104/pp.125.2.711; PMID: 11161028
- Wu JQ, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata.. Plant Cell 2007; 19:1096 - 122; http://dx.doi.org/10.1105/tpc.106.049353; PMID: 17400894
- von Dahl CC, Winz RA, Halitschke R, Kühnemann F, Gase K, Baldwin IT. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata.. Plant J 2007; 51:293 - 307; http://dx.doi.org/10.1111/j.1365-313X.2007.03142.x; PMID: 17559506
- Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, et al. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 2007; 19:2470 - 83; http://dx.doi.org/10.1105/tpc.107.050708; PMID: 17675405
- Giri AP, Wünsche H, Mitra S, Zavala JA, Muck A, Svatos A, et al. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiol 2006; 142:1621 - 41; http://dx.doi.org/10.1104/pp.106.088781; PMID: 17028148
- Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, et al. Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci U S A 2006; 103:8894 - 9; http://dx.doi.org/10.1073/pnas.0602328103; PMID: 16720701
- Alborn HT, Hansen TV, Jones TH, Bennett DC, Tumlinson JH, Schmelz EA, et al. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci U S A 2007; 104:12976 - 81; http://dx.doi.org/10.1073/pnas.0705947104; PMID: 17664416
- Schäfer M, Fischer C, Meldau S, Seebald E, Oelmüller R, Baldwin IT. Lipase activity in insect oral secretions mediates defense responses in Arabidopsis.. Plant Physiol 2011; 156:1520 - 34; http://dx.doi.org/10.1104/pp.111.173567; PMID: 21546453
- Shivaji R, Camas A, Ankala A, Engelberth J, Tumlinson JH, Williams WP, et al. Plants on constant alert: elevated levels of jasmonic acid and jasmonate-induced transcripts in caterpillar-resistant maize. J Chem Ecol 2010; 36:179 - 91; http://dx.doi.org/10.1007/s10886-010-9752-z; PMID: 20148356
- Ribot C, Zimmerli C, Farmer EE, Reymond P, Poirier Y. Induction of the Arabidopsis PHO1;H10 gene by 12-oxo-phytodienoic acid but not jasmonic acid via a CORONATINE INSENSITIVE1-dependent pathway. Plant Physiol 2008; 147:696 - 706; http://dx.doi.org/10.1104/pp.108.119321; PMID: 18434606
- Walter A, Mazars C, Maitrejean M, Hopke J, Ranjeva R, Boland W, et al. Structural requirements of jasmonates and synthetic analogues as inducers of Ca2+ signals in the nucleus and the cytosol of plant cells. Angew Chem Int Ed Engl 2007; 46:4783 - 5; http://dx.doi.org/10.1002/anie.200604989; PMID: 17487903
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010; 468:400 - 5; http://dx.doi.org/10.1038/nature09430; PMID: 20927106
- Ulloa RM, Raíces M, MacIntosh GC, Maldonado S, Téllez-Iñón MT. Jasmonic acid affects plant morphology and calcium-dependent protein kinase expression and activity in Solanum tuberosum. Physiol Plant 2002; 115:417 - 27; http://dx.doi.org/10.1034/j.1399-3054.2002.1150312.x; PMID: 12081535
- Ludwig AA, Romeis T, Jones JD. CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot 2004; 55:181 - 8; http://dx.doi.org/10.1093/jxb/erh008; PMID: 14623901
- Kost C, Heil M. Increased availability of extra foral nectar reduces herbivory in Lima bean plants (Phaseolus lunatus, Fabaceae). Basic Appl Ecol 2005; 6:237 - 48; http://dx.doi.org/10.1016/j.baae.2004.11.002
- War AR, Paulraj MG, War MY, Ignacimuthu S. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal Behav 2011; c 6:1787 - 92; http://dx.doi.org/10.4161/psb.6.11.17685; PMID: 22057329
- Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 2011; 62:3321 - 38; http://dx.doi.org/10.1093/jxb/err031; PMID: 21357767
- Pieterse CMJ, Van Loon LC. NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 2004; 7:456 - 64; http://dx.doi.org/10.1016/j.pbi.2004.05.006; PMID: 15231270
- Peng J, Deng X, Huang J, Jia S, Miao X, Huang Y. Role of salicylic acid in tomato defense against cotton bollworm, Helicoverpa armigera Hubner. Z Naturforsch C 2004; 59:856 - 62; PMID: 15666546
- van Loon LC, Geraats BPJ, Linthorst HJM. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 2006; 11:184 - 91; http://dx.doi.org/10.1016/j.tplants.2006.02.005; PMID: 16531096
- Yujie LU, Xia W, Yonggen L, Jiaan C. Role of ethylene signaling in the production of rice volatiles induced by the rice brown planthopper Nilaparvata lugens.. Chin Sci Bull 2006; 51:2457 - 65; http://dx.doi.org/10.1007/s11434-006-2148-3
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 2002; 5:325 - 31; http://dx.doi.org/10.1016/S1369-5266(02)00275-3; PMID: 12179966
- Kanchiswamy CN, Takahashi H, Quadro S, Maffei ME, Bossi S, Bertea C, et al. Regulation of Arabidopsis defense responses against Spodoptera littoralis by CPK-mediated calcium signaling. BMC Plant Biol 2010; 10:97; http://dx.doi.org/10.1186/1471-2229-10-97; PMID: 20504319
- Smith CM, Boyko EV. The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol Exp Appl 2007; 122:1 - 16; http://dx.doi.org/10.1111/j.1570-7458.2006.00503.x
- Torres MA. ROS in biotic interactions. Physiol Plant 2010; 138:414 - 29; http://dx.doi.org/10.1111/j.1399-3054.2009.01326.x; PMID: 20002601
- Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, et al. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc Natl Acad Sci U S A 2005; 102:10736 - 41; http://dx.doi.org/10.1073/pnas.0502954102; PMID: 16027369
- Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol 2006; 141:384 - 90; http://dx.doi.org/10.1104/pp.106.078295; PMID: 16760492
- Argandoña VH, Chaman M, Cardemil L, Muñoz O, Zúñiga GE, Corcuera LJ. Ethylene production and peroxidase activity in aphid-infested barley. J Chem Ecol 2001; 27:53 - 68; http://dx.doi.org/10.1023/A:1005615932694; PMID: 11382067
- Zheng SJ, Dicke M. Ecological genomics of plant-insect interactions: from gene to community. Plant Physiol 2008; 146:812 - 7; http://dx.doi.org/10.1104/pp.107.111542; PMID: 18316634
- Thompson GA, Goggin FL. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J Exp Bot 2006; 57:755 - 66; http://dx.doi.org/10.1093/jxb/erj135; PMID: 16495409
- Broekgaarden C, Poelman EH, Steenhuis G, Voorrips RE, Dicke M, Vosman B. Genotypic variation in genome-wide transcription profiles induced by insect feeding: Brassica oleracea--Pieris rapae interactions. BMC Genomics 2007; 8:239; http://dx.doi.org/10.1186/1471-2164-8-239; PMID: 17640338
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 2010; 44:1 - 24; http://dx.doi.org/10.1146/annurev-genet-102209-163500; PMID: 20649414
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 2004; 16:3132 - 47; http://dx.doi.org/10.1105/tpc.104.026120; PMID: 15494554
- Zhu-Salzman K, Salzman RA, Ahn JE, Koiwa H. Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol 2004; 134:420 - 31; http://dx.doi.org/10.1104/pp.103.028324; PMID: 14701914
- Voelckel C, Baldwin IT. Herbivore-induced plant vaccination. Part II. Array-studies reveal the transience of herbivore-specific transcriptional imprints and a distinct imprint from stress combinations. Plant J 2004; 38:650 - 63; http://dx.doi.org/10.1111/j.1365-313X.2004.02077.x; PMID: 15125771
- Kempema LA, Cui XP, Holzer FM, Walling LL. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol 2007; 143:849 - 65; http://dx.doi.org/10.1104/pp.106.090662; PMID: 17189325
- Agrawal AA. Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect?. Am Nat 2001; 157:555 - 69; http://dx.doi.org/10.1086/319932; PMID: 18707262
- Agrawal AA. Herbivory and maternal effects: Mechanisms and consequences of transgenerational induced plant resistance. Ecology 2002; 83:3408 - 15; http://dx.doi.org/10.1890/0012-9658(2002)083[3408:HAMEMA]2.0.CO;2
- Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, et al. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One 2010; 5:e9514; http://dx.doi.org/10.1371/journal.pone.0009514; PMID: 20209086
- De Leo F, Bonadé-Bottino M, Ceci LR, Gallerani R, Jouanin L. Effects of a mustard trypsin inhibitor expressed in different plants on three lepidopteran pests. Insect Biochem Mol Biol 2001; 31:593 - 602; http://dx.doi.org/10.1016/S0965-1748(00)00164-8; PMID: 11267898
- Huang W, Zhikuan J, Qingfang H. Effects of herbivore stress by Aphis medicaginis Koch on the malondialdehyde contents and activities of protective enzymes in different alfalfa varieties. Acta Ecol Sin 2007; 27:2177 - 83; http://dx.doi.org/10.1016/S1872-2032(07)60048-1
- Stout MJ, Riggio MR, Yang Y. Direct induced resistance in Oryza sativa to Spodoptera frugiperda.. Environ Entomol 2009; 38:1174 - 81; http://dx.doi.org/10.1603/022.038.0426; PMID: 19689897