Abstract
Carbon translocation in plants is shaped by phyllotaxis and regulated by source/sink interactions that respond to the demands of growth and defense. We have studied this extensively in poplar saplings, and recently showed that unlike carbon import, nitrogen is not translocated to sink leaves in response to application of jasmonic acid. Here we report that this is also true for young trees in the field. We discuss the importance of transport processes in establishing local C:N ratios, and suggest that the JA-induced flow of C but not N to sink tissues, and their corresponding increases in C-based defenses, may simply reflect a plant adaptation to handle excess reduced carbon and energy.
Text
Local increases in sink strength are essential components of plant responses to grazing, mechanical wounding, infection, hormones and artificial elicitors in a wide range of plant species.Citation1 These responses involve rapid increases in the local activities of cell wall invertase enzymes (CWI; EC 3.2.1.26), which boost phloem unloading and carbohydrate (CHO) transport to the elicited tissues.Citation2-Citation8 We have found that both insect grazing and exogenous jasmonate (JA) trigger increased sink strength, including a 3-fold increase in the import of 13C-labeled CHOs from orthostichously linked source leaves to sink leaves in poplar saplings.Citation1,Citation3,Citation4
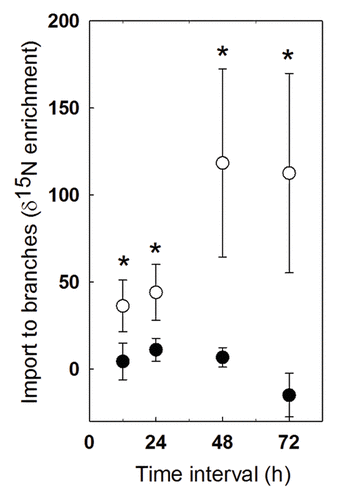
We recently found that unlike carbon import, nitrogen translocation was not increased by JA treatment in poplar saplings.Citation1 We also examined the impact of sink strength on nitrogen translocation in larger, more complex poplar trees, using whole branches, longer transport distances and multiple sampling intervals. We measured 15N flow rates in 4 y-old hybrid poplar trees (OP-367) cultivated at the Dickinson College Agricultural Research Center in Boiling Springs, PA in June and July of 2010. On each tree, two opposing lateral branches were selected and randomly designated as primary (1°) and secondary (2°) branches. The branches were similar, originating at similar truck heights and of similar lengths. For three days, the 1° lateral branches received 5mM JA on the 20 most apical leaves and the 2° branches received nothing. On the morning of day two, we added 15N as a soil drench and harvested leaves 12h, 24h, 48h and 72h later to determine the flow of 15N into 1°and 2° branches. At each interval, two leaves from each 1þand 2þ branch were harvested – one sink leaf and one source leaf – and combined for isotope analysis. Here we found that JA treatment, which generally increases local sink strength, led to significantly lower rates of 15N import at 24h, 48h and 72h compared with rates observed for opposing (untreated) branches on the same trees (). Altogether, we have found that while the supply of CHOs to elicited tissues is related to local sink strength, nitrogen movement is neither related to sink strength nor is it constrained by plant architecture.
Figure 1. Import of 15N to lateral branches of four-year old hybrid poplar trees over a four day period. JA-treated lateral branches did not import additional 15N as a component of the defense responses. In fact, 15N import was lower in these branches (closed circles) compared with control branches located on the same trees (open circles). Points represent the mean import of 4–5 branches with +/1 SE error bars. At each time point, import was compared via two-tailed t-tests. * indicates p < 0.10.
These observations make sense in light of the fact that poplar’s main response to elicitation involves carbon-based metabolites such as polyphenols.Citation9 Increased N transport in response to localized stress has been observed mainly or only in plants that increase production and transport of N-intensive metabolites in response to stress, such as nicotine in tobacco.Citation7,Citation10-Citation13
Taken together, our results indicate that sink-source relationships must frequently determine local CHO/N ratios. Many have noted an inverse relationship in entire plants between N availability and the constitutive concentration of CHO-based metabolites, especially polyphenols.Citation14-Citation16 The relationship between nitrogen availability and phenolic synthesis is regulated at the transcriptional level.Citation17,Citation18A localized carbon excess relative to nutrient availabilities could result from many causes, including the impact of elevated light levels or CO2 concentrations on photosynthesis, heterogeneous vascular connections or changing carbohydrate transport as leaves mature.Citation17,Citation19,Citation20 Since invertase activity is generally influenced by the same factors,Citation3 varying sink strength probably underlies such constitutive variation, which can be ecologically important.Citation19,Citation21-Citation23
But what of elicitation, or “induction” of CHO-based defenses in response to insects or other stresses? Hunter and SchultzCitation16 showed that defoliation-induced increases in polyphenols by oak leaves were suppressed by nitrogen fertilization, as did Keinanen et al.Citation24 for birch and Blodgett et al.Citation25 for pine. Our research has linked short-term, local increases in sink strength via enhanced invertase activity to increased CHO accumulation and an increase in the products of the phenylpropanoid pathway.Citation1,Citation3,Citation4 Like Tuomi et al.Citation22 we also found that this sequence of events is limited to individual branches.Citation1 Since we now see that increased sink strength is associated with no change or even a decrease in local N concentrations, induced sink strength should increase the local CHO/N ratio in elicited tissues. Tuomi et al.Citation22 and Roitto et al.Citation26 suggested that a decrease in N alone would be enough to cause “induction” of polyphenol production in individual branches. Our evidence leads us to suggest that branch-level “induction” of CHO-based compounds in response to insects or other elicitors arises from an increased CHO/N ratio driven by enhanced sink strength. This is consistent with evidence that induced “defense” is a local phenomenon, constrained by vascular architectureCitation27-Citation28 and that local nutrient status can influence both constitutive and inducible defenses.Citation22,Citation27,Citation29,Citation30
Substrates for many putative CHO-based defenses, e.g., flavonoids and tannins, arise from the shikimic acid and phenylpropanoid pathways. These pathways are upregulated by carbon availability and typically respond positively to enhanced carbon supply; they may be a sink for excess reduced carbon and energy.Citation31 According to this view, any photosynthetically active tissue should respond to a real or perceived increase in CHO by shunting excess carbon substrates into these pathways. “Dumping” excess CHO may occur in any tissue that constitutes a strong sink, including fruits, insect galls,Citation32 expanding leavesCitation20 or interrupted nutrient supply,Citation33 and altered “defense” may be an after-effect.
The dynamic interaction between C and N pools in plants and the role of the shikimate and phenylpropanoid pathways as places to sequester excess carbohydrate suggest that we need to re-examine the interpretation of polyphenol accumulation as an active defense. As suggested 30 y agoCitation34,Citation35 for entire plants, it appears to us that the relationship between N and C availability also determines how phenylpropanoids accumulate at the local level and that sink strength forms the driving force in “induced” responses to elicitors. Phenylpropanoid production may be an adaptive way to dispose of carbohydrates and energy that have accumulated in excess of the tissue’s ability to use them in growth or maintenance when sink strength is increased.
| Abbreviations: | ||
| C | = | carbon |
| CHO | = | carbohydrate |
| JA | = | jasmonate |
| CWI | = | cell wall invertase |
| N | = | nitrogen |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Appel HM, Arnold TM, Schultz JC. Effects of jasmonic acid, branching and girdling on carbon and nitrogen transport in poplar. New Phytol 2012; 195:419 - 26; 10.1111/j.1469-8137.2012.04171.x; PMID: 22621389
- Kleiner KW, Raffa KF, Dickson RE. Partitioning of 14C-labeled photosynthate to allelochemicals and primary metabolites in source and sink leaves of aspen: evidence for secondary metabolite turnover. Oecologia 1999; 119:408 - 18; 10.1007/s004420050802
- Arnold TM, Schultz JC. Induced sink strength as a prerequisite for induced tannin biosynthesis in developing leaves of Populus.. Oecologia 2002; 130:585 - 93; 10.1007/s00442-001-0839-7
- Arnold TM, Appel HM, Patel V, Stocum E, Kavalier A, Schultz JC. Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink–source model of plant defense. New Phytol 2004; 164:157 - 64; 10.1111/j.1469-8137.2004.01157.x
- Matyssek R, Agerer R, Ernst D, Munch J-C, Osswald W, Pretzsch H, et al. The plant’s capacity in regulating resource demand. Plant Biol (Stuttg) 2005; 7:560 - 80; 10.1055/s-2005-872981; PMID: 16388460
- Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, et al. Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus.. New Phytol 2005; 167:63 - 72; 10.1111/j.1469-8137.2005.01388.x; PMID: 15948830
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci U S A 2006; 103:12935 - 40; 10.1073/pnas.0602316103; PMID: 16912118
- Newingham BA, Callaway RM, Bassirirad H. Allocating nitrogen away from a herbivore: a novel compensatory response to root herbivory. Oecologia 2007; 153:913 - 20; 10.1007/s00442-007-0791-2; PMID: 17619205
- Lindroth RL, Hwang SY. Clonal variation in foliar chemistry of quaking aspen (Populus tremuloides Michx.). Biochem Syst Ecol 1996; 24:357 - 64; 10.1016/0305-1978(96)00043-9
- Beardmore T, Wetzel S, Kalous M. Interactions of airborne methyl jasmonate with vegetative storage protein gene and protein accumulation and biomass partitioning in Populus plants. Can J Res 2000; 30:1106 - 13
- Rossato L, MacDuff JH, Laine P, Le Deunff E, Ourry A. Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: effects of methyl jasmonate on nitrate uptake, senescence, growth, and VSP accumulation. J Exp Bot 2002; 53:1131 - 41; 10.1093/jexbot/53.371.1131; PMID: 11971924
- Meuriot F, Avice J-C, Decau M-L, Simon J-C, Lainé P, Volenec JJ, et al. Accumulation of N reserves and vegetative storage protein (VSP) in taproots of non-nodulated alfalfa (Medicago sativa L.) are affected by mineral N availability. Plant Sci 2003; 165:709 - 18; 10.1016/S0168-9452(03)00225-5
- Gómez S, Ferrieri RA, Schueller M, Orians CM. Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol 2010; 188:835 - 44; 10.1111/j.1469-8137.2010.03414.x; PMID: 20723074
- Ruan J, Haerdter R, Gerendás J. Impact of nitrogen supply on carbon/nitrogen allocation: a case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol (Stuttg) 2010; 12:724 - 34; 10.1111/j.1438-8677.2009.00288.x; PMID: 20701695
- Glynn C, Herms DA, Egawa M, Hansen R, Mattson WJ. Effects of nutrient availability on biomass allocation as well as constitutive and rapid induced herbivore resistance in poplar. Oikos 2003; 101:385 - 97; 10.1034/j.1600-0706.2003.12089.x
- Hunter MD, Schultz JC. Fertilization mediates within-tree phytochemical induction by the gypsy moth in oaks. Ecology 1995; 76:1226 - 32; 10.2307/1940929
- Harding SA, Jarvie MM, Lindroth RL, Tsai CJ. A comparative analysis of phenylpropanoid metabolism, N utilization, and carbon partitioning in fast- and slow-growing Populus hybrid clones. J Exp Bot 2009; 60:3443 - 52; 10.1093/jxb/erp180; PMID: 19516073
- Lillo C, Lea US, Ruoff P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 2008; 31:587 - 601; 10.1111/j.1365-3040.2007.01748.x; PMID: 18031469
- Orians C, Jones CG. Plants as resource mosaics: a functional model for predicting patterns of within-plant resource heterogeneity to consumers based on vascular architecture and local environmental variability. Oikos 2001; 94:493 - 504; 10.1034/j.1600-0706.2001.940311.x
- Coley PD, Kursar TA. Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological tradeoffs. Pages 305-336 In: Tropical Forest Plant Ecophysiology, edited by SS Mulkey, R. Chazdon and AP Smith. Chapman and Hall, NY. 1996: 675 pp.
- Denno RF, McClure MS, eds. Variable plants and herbivores in natural and managed systems. Academic Press, New York. 1983: 717pp.
- Tuomi J, Niemaelä P, Rosi M, Sirèn S, Vuorisalo T. Induced accumulation of foliage phenols in mountain birch: branch response to defoliation?. Am Nat 1988; 132:602 - 8; 10.1086/284875
- Suomela J, Kaitaniemi P, Nilson A. Systematic within-tree variation in mountain birch leaf quality for a geometrid, Epirritaautumnata. Ecol Entomol 1995; 20:283 - 92; 10.1111/j.1365-2311.1995.tb00458.x
- Keinanen M, Julkunen-Tiitto R, Mutikain P, Walls M, Ovaska J, Vapaavuori E. Trade-offs in phenolic metabolism of silver birch: effects of fertilization, defoliation and genotype. Ecology 1999; 80:1970 - 86
- Blodgett JT, Herms DA, Bonello P. Effects of fertilization on red pine defense chemistry and resistance to Sphaeropsis sapinea.. For Ecol Manage 2005; 208:373 - 382; 10.1016/j.foreco.2005.01.014
- Roitto M, Rautio P, Markkola A, Julkunen-Tiitto R, Varama M, Saravesi K, et al. Induced accumulation of phenolics and sawfly performance in Scots pine in response to previous defoliation. Tree Physiol 2009; 29:207 - 16; 10.1093/treephys/tpn017; PMID: 19203946
- Tuomi J, Niemelä P, Fagerström T. Nutrient stress: An explanation for plant anti-herbivore responses to defoliation. Oecologia 1984; 61:208 - 10; 10.1007/BF00396762
- Orians CM, Ardón M, Mohammad BA. Vascular architecture and patchy nutrient availability generate within-plant heterogeneity in plant traits important to herbivores. Am J Bot 2002; 89:270 - 8; 10.3732/ajb.89.2.270; PMID: 21669736
- Haukioja E. Induction of defenses in trees. Annu Rev Entomol 1991; 36:25 - 42; 10.1146/annurev.en.36.010191.000325
- Honkanen T, Haukioja E, Suomela J. Effects of simulated defoliation and debudding on needle and shoot growth in Scots pine (Pinussylvestris): implications of plant source/sink relationships for plant-herbivore studies. Funct Ecol 1994; 8:631 - 9; 10.2307/2389926
- Hernández I, Van Breusegem F. Opinion on the possible role of flavonoids as energy escape valves: novel tools for nature’s Swiss army knife?. Plant Sci 2010; 179:297 - 301; 10.1016/j.plantsci.2010.06.001
- Rehill BJ, Schultz JC. Enhanced invertase activities in the galls of Hormaphis hamamelidis.. J Chem Ecol 2003; 29:2703 - 20; 10.1023/B:JOEC.0000008014.12309.04; PMID: 14969357
- Steele L, Caldwell M, Boettcher A. Arnold, T. Seagrass–pathogen interactions: ‘pseudo-induction’ of turtlegrass phenolics near wasting disease lesions. Mar Ecol Prog Ser 2005; 303:123 - 31; 10.3354/meps303123
- Bryant JP, Chapin FS III, Klein DR. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory (hare, Lepuscapensis). Oikos 1983; 40:357 - 68; 10.2307/3544308
- Coley PD, Bryant JP, Chapin FS 3rd. Resource availability and plant antiherbivore defense. Science 1985; 230:895 - 9; 10.1126/science.230.4728.895; PMID: 17739203