Abstract
RNA silencing inducible sequence (RSIS) causes post-transcriptional gene silencing (PTGS) of 5' or 3' flanking sequence-containing genes by inhibiting proper transcriptional termination. Exploiting this nature, 13 kD Pro-less, in which major seed storage protein (SSP) 13 kD prolamins are reduced, has been generated. In 13 kD Pro-less, other SSPs, such as glutelins, are increased as a compensation effect to maintain amino acid pool. 7Crp is the seven-linked epitope peptide derived from major cedar pollen allergens Cry j 1 and Cry j 2. When 7Crp is expressed in 13 kD Pro-less endosperm, accumulation level of 7Crp increased. Furthermore, recovery rate of 7Crp without reducing reagent increased. These findings indicate that 13 kD Pro-less endosperm provides a good production platform for recombinant proteins.
RNA silencing inducible sequence (RSIS) is about 90bp artificial sequence encoding modified human Glucagon-like peptide-1 (mGLP-1).Citation1,Citation2RSIS induces nuclei post-transcriptional gene silencing (PTGS) of 5′ or 3′ flanking sequence-containing genes in rice. This is due to the nature of RSIS inhibiting proper transcriptional termination.Citation1 To evaluate the usefulness of this RNA silencing method, we have generated several seed storage protein (SSP) knock-down lines.Citation3 When one or a few SSP(s) was reduced, other SSPs increased and compensated for the reduced SSP at both the mRNA and protein levels.Citation3 In addition, formulation of storage organelle, where SSPs are deposited, was altered.Citation3 Seed is one of the most popular tissues to accumulate valuable recombinant proteins/peptides.Citation4 Previously we have shown that mutants lacking some glutelins accumulate more recombinant proteins.Citation5 Here, we examined how recombinant protein production is affected in two SSP knockdown lines, GluB-less and 13 kD Pro-less, in which GluB-1 and 13kD prolamin genes were suppressed by using RSIS. As a reference, we used a transgenic rice accumulating 7Crp, the seven-linked epitope peptide derived from major cedar pollen allergens Cry j 1 and Cry j 2.Citation6
RSIS Induces Silencing of Transgene
We crossed 7Crp rice and GluB-less, and analyzed the relation between 7Crp accumulation and GluB-1 accumulation in F2 generation. Unexpectedly, 7Crp was not accumulated in GluB-less background, whereas 7Crp accumulated in wild type background (data not shown), indicating that 7Crp was silenced because 7Crp mRNA contains 5′ and 3′ UTRs of GluB-1. Thus, we concluded that RSIS induces silencing of transgenes as well as endogenous genes. This raises a future feasibility of reporter system, such as using luciferase and GFP, to analyze the mechanism of RSIS-mediated silencing.
Thirteen kD Pro-less is a Good Production Platform
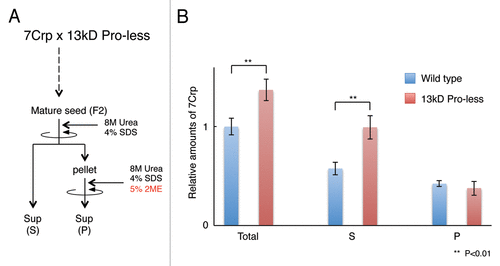
We crossed 7Crp rice and 13 kD Pro-less, and analyzed the relation between 7Crp accumulation and 13 kD prolamins accumulation in F2 generation. In 13 kD Pro-less background, 7Crp accumulation increased by 1.4-fold of that in wild type background (). This may be due to compensation effect, because GluB-1 is upregulated in 13 kD Pro-less and 7Crp was driven by the GluB-1 promoter. 7Crp is predominantly deposited in endoplasmic reticulum-derived storage organelle PB-I where prolamins are also deposited. 7Crp has a free cysteine residue and easily makes disulfide-bond with cysteine-rich prolamins.Citation7 Therefore, 7Crp is difficult to be extracted without reducing reagent.Citation7 If recombinant proteins should have bioactivities, easy and high-yield extractability without reducing reagent is desired.Citation8 To examine whether extractability is improved by reducing 13 kD prolamins, we extracted proteins (S) without reducing reagent, then extracted residual proteins (P) with reducing reagent (). 7Crp in S fraction in 13 kD Pro-less background increased by 1.7-fold of that in wild type background (). In contrast, 7Crp in P fraction were similar between in wild type background and in 13 kD Pro-less background (). These results suggest that extractability of 7Crp without reducing reagent was improved in 13 kD Pro-less.
Figure 1. 7Crp accumulation level and its extractability without reducing reagent. (A) A scheme of experiment. Seed proteins are extracted with 8M urea and 4% SDS buffer. This fraction extractable without reducing reagent is designated as (S). Residual proteins (P) are extracted with 8M urea, 4% sodium dodecyl sulfate (SDS), and 5% 2-mercaptoethanol (2ME) as reducing reagent. (B) 7Crp levels in each fraction. Total is calculated as sum of (S) and (P). ** indicates significant difference p < 0.01 between in wild type background and in 13 kD Pro-less background (Student’s t-test).
We showed accumulation level of 7Crp and its recovery rate without reducing reagent increased in 13 kD Pro-less. This approach will be applicable to production of other recombinant proteins, and accelerate practical use of transgenic rice as production platform of recombinant proteins.
| Abbreviations: | ||
| PTGS | = | post-transcriptional gene silencing |
| RSIS | = | RNA silencing inducible sequence |
| SSP | = | seed storage protein |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by research grants from the Ministry of Agriculture, Forest and Fishery of Japan (Genomics and Agricultural Innovation: GMC0003) and by a Grant-in-Aid for Young Scientists (22688001) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Kawakatsu T, Wakasa Y, Yasuda H, Takaiwa F. RNA silencing induced by an artificial sequence that prevents proper transcription termination in rice. Plant Physiol 2012; In press 10.1104/pp.112.202689; PMID: 22843666
- Yasuda H, Tada Y, Hayashi Y, Jomori T, Takaiwa F. Expression of the small peptide GLP-1 in transgenic plants. Transgenic Res 2005; 14:677 - 84; 10.1007/s11248-005-6631-4; PMID: 16245158
- Kawakatsu T, Hirose S, Yasuda H, Takaiwa F. Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiol 2010; 154:1842 - 54; 10.1104/pp.110.164343; PMID: 20940349
- Kawakatsu T, Takaiwa F. Cereal seed storage protein synthesis: fundamental processes for recombinant protein production in cereal grains. Plant Biotechnol J 2010; 8:939 - 53; 10.1111/j.1467-7652.2010.00559.x; PMID: 20731787
- Tada Y, Utsumi S, Takaiwa F. Foreign gene products can be enhanced by introduction into low storage protein mutants. Plant Biotechnol J 2003; 1:411 - 22; 10.1046/j.1467-7652.2003.00038.x; PMID: 17134400
- Takagi H, Saito S, Yang L, Nagasaka S, Nishizawa N, Takaiwa F. Oral immunotherapy against a pollen allergy using a seed-based peptide vaccine. Plant Biotechnol J 2005; 3:521 - 33; 10.1111/j.1467-7652.2005.00143.x; PMID: 17173638
- Takaiwa F, Hirose S, Takagi H, Yang L, Wakasa Y. Deposition of a recombinant peptide in ER-derived protein bodies by retention with cysteine-rich prolamins in transgenic rice seed. Planta 2009; 229:1147 - 58; 10.1007/s00425-009-0905-7; PMID: 19247688
- De Muynck B, Navarre C, Boutry M. Production of antibodies in plants: status after twenty years. Plant Biotechnol J 2010; 8:529 - 63; 10.1111/j.1467-7652.2009.00494.x; PMID: 20132515