Abstract
Plant defensive behaviors that resist arthropod herbivory include trichome-mediated defenses, and variation in plant trichome morphology and abundance provides examples of the mechanistic complexities of insect-plant interactions. Trichomes were removed from Cycas revoluta cataphylls on the island of Guam to reveal Aulacaspis yasumatsui scale infestation, and predation of the newly exposed insects by pre-existing Rhyzobius lophanthae beetles commenced within one day. The quotient of predated/total scale insects was 0.5 by day 4 and stabilized at that found on adjacent glabrous leaves in about one week. The trichome phenotype covering the C. revoluta cataphyll complex offers the invasive A. yasumatsui armored scale effectual enemy-free space in this system. This pest and predator share no known evolutionary history with C. revoluta, therefore, the adaptive significance of this plant behavior in natural habitat is not yet known.
Introduction
The invasion of Guam by Aulacaspis yasumatsui was predicted in 2000 and verified in Oct. 2003 as a limited infestation in the landscape of the main hotel district, then dispersal of the pest from urban areas to native forest habitat was documented in Jan. 2005.Citation1 The scale predator Rhyzobius lophanthae was purposely introduced in Nov. 2004, and released in native forest habitats beginning Feb. 2005.Citation2 This predator rapidly established and signs of scale predation have been persistent. Despite this history, plant mortality has been epidemic and continues to date. Only a few of the Cycas revoluta trees within urban forests are still alive, and more than 90% of the native Cycas micronesica trees have been killed by the chronic scale infestations.Citation3
After full establishment of the predator, the scale individuals that lacked predation were many times located in small crevices or other locations that appeared to be too diminutive for the predator to maneuver. In attempts to validate this observation, Cycas revoluta cataphyll surfaces were exposed by removing the very dense trichomes () that cover this taxon’s cataphylls.Citation4 As expected, the newly exposed plant surfaces revealed heavy infestations of cryptic scale showing no signs of predation despite the presence of Rhyzobius lophanthae beetles on the same plants.
Figure 1. The Cycas revoluta cataphyll complex is adorned with elaborate tufts of trichomes (top). Quotient of Aulacaspis yasumatsui individuals on Cycas revoluta cataphylls that were predated by Rhyzobius lophanthae beetles as a function of days after trichome removal. Mean ± SE (bottom).
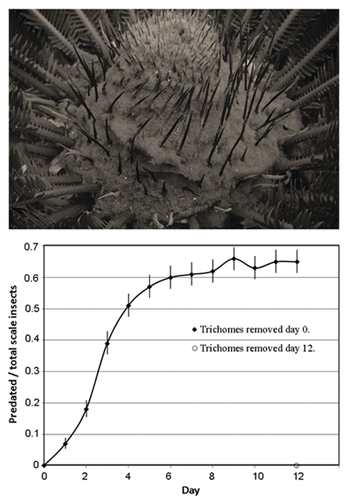
Trichome morphology per se was clearly unable to deter scale infestations on the cataphyll surfaces, and we hypothesized that trichome exclusion of R. lophanthae was responsible for the complete lack of biological control of the scale insects infesting these trichome-endowed plant surfaces.Citation4 Here I tested this hypothesis by exposing A. yasumatsui on cataphyll surfaces via removal of trichomes, then monitoring predation of the scales that were exposed until predation percentage reached that of the scale infestations on leaf surfaces from the same plants.
Results
The interactive effect of date X time of day was not significant (p = 0.15), and the main factor time of day was not significant (p = 0.25) for the number of beetles observed on the exposed half of the cataphyll complex. The number of beetles observed differed among the dates (f = 9.20, df = 12, p < 0.0001). No beetles were searching for or feeding on scales on the newly exposed cataphyll surfaces on the initial date, but beetles were observed beginning 0800 h on the next day. Therefore, less than 24 h was needed for this predator to move into the space exhibiting newly exposed prey. The number of beetles increased daily until peaking on day 4 at 2.44 per plant, then gradually declined until day 9 when the counts stabilized at ca 0.4 per plant.
The interactive effect of date X sex was not significant (p = 0.98), and the main factor time of sex was not significant (p = 0.70) for the quotient of predated/total scale insects. However, the quotient of predated/total scale insects differed among the dates (f = 27.46, df = 12, p < 0.0001). Predation quotient was almost 0.1 by day 1, and rapidly increased to 0.5 by day 4 (). The scale predation reached a stable ca. 0.65 by day 8 or 9.
Trichomes were removed on day 12 from the second half of each cataphyll complex. There were ca. 4 scales per cm2, and number of R. lophanthae and predation were nil () as with day 0 for the initially exposed half.
Discussion
The results of this study verify that the ability of A. yasumatsui to infest trichome-protected cataphylls on C. revoluta plants on Guam is a direct result of preferential exclusion of the R. lophanthae predators by the trichomes. Predators were observed feeding on A. yasumatsui within one day of cessation of the prophylactic behavior provided by trichomes. Moreover, the quotient of predated/total A. yasumatsui individuals reached that of adjacent glabrous leaflet surfaces, which are not protected by heavy trichome density, in about one week following trichome removal.
Structural defenses employed by plants to resist arthropod herbivory include trichome-mediated defenses, and numerous studies validate that trichomes reduce damage caused by herbivorous insects on some plant species.Citation5-Citation11 However, host plant phenotype plays a crucial role in how herbivorous arthropods interact with entomophagous arthropods.Citation12 Some studies reveal no effect of trichome phenotype or density on performance of natural enemies of herbivorous insects.Citation13 Alternatively, other studies reveal that trichomes reduce the extent of insect herbivory by aiding predators or parasitoids.Citation14 Similar to my results, other studies reveal that trichomes increase extent of insect herbivory by decreasing performance of predators or parasitoidsCitation15-Citation20 and some of these included Coccinelidae predators.Citation21,Citation22
In this case study from the Mariana Islands, the Cycas tree population is only recently interacting in an anthropogenic-initiated tri-trophic quagmire. The alien herbivore pressure was initiated in 2003 by an unintentional introduction,Citation1,Citation23 and the alien predator protection was initiated in 2005 by a purposeful introduction.Citation2 Within the context of this artificial tri-trophic system, the ability of C. revoluta to protect plant surfaces with trichomes is disadvantageous, offering the lethal herbivore physical protection from predation.
General observations indicate trichome morphotype is a stable trait among cycad taxa and is therefore useful for taxonomy.Citation24 However, the adaptive significance of trichome production within the Cycas genus is not known. Trichomes serve purposes other than direct influence on insect herbivores and their natural enemies. For example, trichomes may increase tolerance of abiotic factors such as drought stress and UV exposure, and help regulate organ temperatures.Citation25,Citation26 Furthermore, foliar trichomes have been shown to express highly efficient uptake of nitrogen,Citation27 phosphorus,Citation28 and potassium.Citation29 Production of trichomes by Cycas plants may fulfill one of these alternative roles with my observed effects on excluding a specialist pest’s predator emerging as an incidental behavior.
This paper points out the uncertainties of using generalist alien predators for control of specialist alien arthropod pests. Perhaps biological control programs that rely on generalist predators would best be served with an expectation that initial control may not be entirely effective. This expectation may enable a more aggressive initial search for the factors that reduce effectiveness, followed by a vigorous search for alternative biological control agents that do not share the defined limitations of the initial biological control organism. In this case a parasitoid that has a body size similar to scale crawlers may not be hindered by the same prophylactic exclusion imposed by the dense trichome display of C. revoluta. Following the A. yasumatsui invasion of Guam we introduced R. lophanthae because it is known as one of the most economically important natural enemies of armored scale insectsCitation30 and pre-existing populations established as a predator on A. yasumatsui after the scale invaded FloridaCitation31 and Texas.Citation32 We could have been more aggressive in continuing the search for a second biological control organism at the onset if we had been more astute in predicting the Cycas plant behaviors that have denied effective R. lophanthae predation over time. The results of my study indicate the introduction and release of a second Coccinelidae predator would likely be constrained by the same size differential that disallows effective R. lophanthae control on the surfaces of many Cycas organs. Biological control goals may be reached more effectively if efforts are directed toward continued search for a parasitoid with a body size that would be able to maneuver into the cryptic locations that are inaccessible to the predator.
Materials and methods
Cycas revoluta cataphyll trichomes were removed by water spray as previously described.Citation4 I removed the trichome cover on one half of the cataphyll complex for each of six trees, leaving the opposite half with intact trichomes. Following this procedure I counted the number of R. lophanthae visible on the surface of the exposed cataphylls then counted the number of male scale and female scales within ten randomly placed 5x5 mm squares as previously described.Citation4 I also recorded if each scale had been predated by R. lophanthae. The beetle counts were repeated at 0800 h, 1200 h, and 1800 h beginning the following day and continuing until day 12. I repeated the scale counts and determination of predation at 0800 h every day.
The beetle count data were square-root transformed (count + 0.0001) and the P values for main factors date, time of day, and the interaction between date and time of day were obtained by fitting a Generalized Linear Mixed Model (SAS Procedure GLIMMIX) with a Poisson distribution. The response variable used to quantify predation was the quotient predated scales/total scales. This variable was square-root transformed and the data were fitted with a Generalized Linear Mixed Model with Poisson distribution. The main factors were date, sex, and the interaction of date and sex.
On the final day of measurements, I removed the trichomes from the second half of each cataphyll complex to validate that increased predation on the initially exposed half was indeed due to the experimental treatment and not coincidental timing of increased predation. Following exposure of new cataphyll surfaces, I conducted the beetle counts, scale counts, and determination of predation.
Conclusions
Studying variation in plant trichome morphology and abundance in plant surface morphology provides examples of the mechanistic complexities of insect-plant interactions. The C. revoluta trichomes clearly defended the cataphyll surfaces against access by an arthropod. Unfortunately, that arthropod was an entomophagous predator. Therefore, the trichome phenotype covering the C. revoluta cataphyll complex exhibited a boomerang effect, where the invasive A. yasumatsui armored scale used the plant’s structural defense traits to find enemy-free space. To my knowledge, this is the first report on the influence of trichomes on plant-herbivore-predator interactions for any cycad species. Trichome phenotype and density may be involved in mediating invasive insect damage to some of the other 331 described cycad species,Citation33 which collectively comprise the most threatened group of plants worldwide.Citation34 A. yasumatsui and R. lophanthae share no known evolutionary history with C. revoluta, so the adaptive significance of this plant behavior in natural habitat is not yet known.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank George Fernandez for statistical analysis. Support provided by US Forest Service Project No. 10-DG-11059702-095. This material was made possible, in part, by a Cooperative Agreement from the United States Department of Agriculture's Animal and Plant Health Inspection Service (APHIS). It may not necessarily express APHIS' views
References
- Terry I, Marler T. Paradise lost? Tipping the scales against Guam’s Cycas micronesica.. The Cycad Newsletter 2005; 28:21 - 3
- Moore A, Marler T, Miller RH, Muniappan R. Biological control of cycad aulacaspis scale on Guam. The Cycad Newsletter 2005; 28:6 - 8
- Marler TE, Lawrence JH. Demography of Cycas micronesica on Guam following introduction of the armoured scale Aulacaspis yasumatsui.. J Trop Ecol 2012; 28:233 - 42; 10.1017/S0266467412000119
- Marler TE, Moore A. Cryptic scale infestations on Cycas revoluta facilitate scale invasions. HortScience 2010; 45:837 - 9
- Ågren J, Schemske DW. The cost of defense against herbivores: an experimental study of trichome production in Brassica rapa.. Am Nat 1993; 141:338 - 50; 10.1086/285477; PMID: 19426086
- Baur R, Binder S, Benz G. Nonglandular leaf trichomes as short-term inducible defense of the gray alder, Alnus incana (L.), against the chysomelid beetle, Agelastica alni L. Oecologia 1991; 87:219 - 26; 10.1007/BF00325259
- Farrar RR, Kennedy GG. Insect and mite resistance in tomato. In: Kalloo G, ed. Genetic improvement of tomato. Monographs on theoretical and applied genetics. Vol 14, Berlin, Springer-Verlag, 1991:121-42.
- Handley RJ, Ekbom B, Ågren J. Variation in trichome density and resistance against a specialist herbivore in natural populations of Arabidopsis thaliana.. Ecol Entomol 2005; 30:284 - 92; 10.1111/j.0307-6946.2005.00699.x
- Løe G, Toräng P, Gaudeul M, Ågren J. Trichome production and spatiotemporal variation in herbivory in the perennial herb Arabidopsis lyrata.. Oikos 2007; 116:134 - 42; 10.1111/j.2006.0030-1299.15022.x
- Simmons AT, Gurr GM. BcGrath D, Nicol HI, Martin PM. Trichomes of Lycopersicon spp. and their effect on Myzus persicae.. Aust J Entomol 2003; 42:373 - 8; 10.1046/j.1440-6055.2003.00376.x
- Valverde PL, Fornoni J, Nuñez-Farfán J. Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium.. J Evol Biol 2001; 14:424 - 32; 10.1046/j.1420-9101.2001.00295.x
- Cortesero AM, Stapel JO, Lewis WJ. Understanding and manipulating plant attributes to enhance biological control. Biol Control 2000; 17:35 - 49; 10.1006/bcon.1999.0777
- Obrycki JJ, Tauber MJ, Tingey WM. Predator and parasitoid interaction with aphid-resistant potatoes to reduce aphid densities: a two-year field study. J Econ Entomol 1983; 74:456 - 62
- Voigt D, Gorb E, Gorb S. Plant surface-bug interactions: Dicyphus errans stalking along trichomes. Arthropod-Plant Interact 2007; 1:221 - 43; 10.1007/s11829-007-9021-4
- Barbour JD, Farrar RR Jr., Kennedy GG. Interaction of Manduca sexta resistance in tomato with insect predators of Helicoverpa zea.. Entomol Exp Appl 1993; 68:143 - 55; 10.1111/j.1570-7458.1993.tb01697.x
- Kauffman WC, Kennedy GG. Relationship between trichome density in tomato and parasitism of Heliothis spp. (Lepidoptera: Noctuidae) eggs by Trichogramma spp. (Hymenoptera: Trichogrammatidae). Environ Entomol 1989; 18:698 - 704
- Krips OE, Kleijn PW, Willems PEL, Gols GJZ, Dicke M. Leaf hairs influence searching efficiency and predation rate of the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). Exp Appl Acarol 1999; 23:119 - 31; 10.1023/A:1006098410165
- McAuslane HJ, Johnson FA, Colvin DL, Sojack B. Influence of foliar pubescence on abundance and parasitism of Bemesia argentifolia (Homoptera: Aleyrodidae) on soybean and peanut. Environ Entomol 1995; 24:1135 - 43
- Treacy MF, Zummo GR, Benedict JH. Interactions of host-plant resistance in cotton with predators and parasites. Agric Ecosyst Environ 1985; 13:151 - 7; 10.1016/0167-8809(85)90057-X
- van Lenteren JC, Hua LZ, Kamerman JW, Rumei X. The parasite-host relationship between Encarsia formosa (Hym., Aphelinidae) and Trialeurodes vaporariorum (Hom., Aleyrodidae) XXVI. Leaf hairs reduce the capacity of Encarsia to control greenhouse whitefly on cucumber. J Appl Entomol 2009; 119:553 - 9; 10.1111/j.1439-0418.1995.tb01335.x
- Eisner T, Eisner M, Hoebeke ER. When defense backfires: detrimental effect of a plant’s protective trichomes on an insect beneficial to the plant. Proc Natl Acad Sci U S A 1998; 95:4410 - 4; 10.1073/pnas.95.8.4410; PMID: 9539750
- Lucas E, Labrecque C, Coderre D. Delphastus catalinae and Coleomegilla maculata lengi (Coleoptera: Coccinellidae) as biological control agents of the greenhouse whitefly, trialeurodes vaporariorum (Homoptera: Aleyrodidae). Pest Manag Sci 2004; 60:1073 - 8; 10.1002/ps.916; PMID: 15532680
- Marler TE, Muniappan R. Pests of Cycas micronesica leaf, stem, and male reproductive tissues with notes on current threat status. Micronesica 2006; 39:1 - 9
- Stevenson DW. Observations on ptyxis, phenology, and trichomes in the Cycadales and their systematic implications. Am J Bot 1981; 68:1104 - 14; 10.2307/2442720
- Ehleringer J. Ecology and ecophysiology of leaf pubescence in North American desert plants. In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes; Plenum Press, New York, 1984:113-32.
- Wagner GJ, Wang E, Shepherd RW. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot 2004; 93:3 - 11; 10.1093/aob/mch011; PMID: 14678941
- Inselsbacher E, Cambui CA, Richter A, Stange CF, Mercier H, Wanek W. Microbial activities and foliar uptake of nitrogen in the epiphytic bromeliad Vriesea gigantea.. New Phytol 2007; 175:311 - 20; 10.1111/j.1469-8137.2007.02098.x; PMID: 17587379
- Winkler U, Zotz G. Highly efficient uptake of phosphorus in epiphytic bromeliads. Ann Bot 2009; 103:477 - 84; 10.1093/aob/mcn231; PMID: 19033287
- Winkler U, Zotz G. ‘And then there were three’: highly efficient uptake of potassium by foliar trichomes of epiphytic bromeliads. Ann Bot (Lond) 2010; 106:421 - 7; 10.1093/aob/mcq120
- Rosen D, ed. Armored scale insects: their biology, natural enemies and control. Elsevier, Amsterdam. 1990; 688.
- Cave RD. Biological control agents of the cycad aulacaspis scale, Aulacaspis yasumatsui.. Proc Fla State Hort Soc 2006; 119:422 - 4
- Flores D, Carlson J. Fortuitous establishment of Rhyzobius lophanthae (Coleoptera: Coccinellidae) and Aphytis lingnanesis (Hymenoptera: Encyrtidae) in South Texas on the cycad aulacaspis scale, Aulacaspis yasumatsui (Hemiptera: Diaspididae). Southwest Entomologist 2009; 34:489 - 92; 10.3958/059.034.0413
- Osborne R, Calonje M, Hill K, Stanberg L, Stevenson DW. The World List of Cycads. Mem N Y Bot Gard 2012; 106:480 - 508
- Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, et al. The impact of conservation on the status of the world’s vertebrates. Science 2010; 330:1503 - 9; 10.1126/science.1194442; PMID: 20978281