Abstract
The TIM17:23 complex on the mitochondrial inner membrane is responsible for import of the majority of mitochondrial proteins in plants. In Arabidopsis, Tim17 and Tim23 belong to a large gene family consisting of 16 members termed the Preprotein and Amino acid transporters (PRAT). Recently, two members of this protein family, Tim23-2 and the Complex I subunit B14.7, have been shown to assemble into both Complex I of the respiratory chain and the TIM17:23 complex (Wang et al., 2012), adding to other examples of links between respiratory and protein import complexes. These associations provide a mechanism to coordinate mitochondrial activity and biogenesis.
Preface
There is a increasing number of reports detailing interactions between protein complexes involved in respiration and protein import in yeast (Saccharomyces cerevisiae) and plants. While the biological significance of these reports has remained unclear, the recent report of an interaction between Complex I of the respiratory chain in Arabidopsis (Arabidopsis thaliana) and TIM17:23 complex points to an important role in coordinating mitochondrial biogenesis with mitochondrial function. However, before proceeding with summarizing these interactions, it is important to clarify some definitions with respect to this topic.
Complex – an assembly of several proteins that have been characterized biochemically, where the subunits are defined to occur in the complex, in a defined stoichiometry.
Super-complex – Two complexes that are physically associated, usually evidenced by blue native-PAGE (BN-PAGE) analysis.
Associated protein – A protein that can import and assemble into a complex, but not in a defined stoichiometry, i.e., it may only be present in a fraction of the protein complex.
Super-complexes and interactions between respiratory and prepreprotein translocase complexes
The limited coding capacity of mitochondria means that the majority of the mitochondrial proteome is encoded by nuclear located genes, which after transcription, are translated in the cytosol, then imported and assembled into the correct location in the mitochondrion. Large multi-subunit protein complexes consisting of preprotein receptors and translocator channels located on the outer and inner mitochondrial membranes are responsible for the import of cytoplasmically synthesized precursor proteins. The majority of proteins that constitute the respiratory chain complexes are encoded by nuclear located genes and thus are imported via these protein transporters. It has been shown that there is a link between the mitochondrial protein import apparatus and the respiratory chain in Arabidopsis.Citation1 The translocase of the inner membrane 23 (Tim23) is a component of the inner membrane channel responsible for the import of the majority of the matrix proteome. Tim23–2 was shown to be part of the TIM17:23 complex of the inner membrane, in agreement with a variety of studies that identify it as a component of this protein complex.Citation1,Citation2 However, recently Tim23–2 was also shown to associate with the monomeric form of respiratory chain Complex I in Arabidopsis.Citation1 In addition, the Complex I subunit B14.7,Citation3,Citation4 which displays similarity with Tim23, was found associate with the TIM17:23 complex.Citation1 In addition, TIM17:23 was also found to interact with the respiratory Complex III via Tim21, similar to an interaction that had previously been identified in yeast, the widely used model system to study mitochondrial protein import.Citation5 The association of TIM17:23 with Complex III was proposed to allow for the TIM17:23 complex to switch between two forms, for translocation across the inner membrane or sorting of protein into the inner membrane by the stop-transfer mechanism.Citation5,Citation6 Furthermore, this association could also be to increase the import efficiency of protein import, by utilizing the strong proton gradient that may exist in the microenvironment, and also maintain contact sites and cristae junctions.
In Arabidopsis, B14.7 and Tim23 are members of the Preprotein and Amino Acid Transporter family (PRAT).Citation7,Citation8 This family of 16 proteins are proposed to be derived from a single eubacterial ancestor and comprise of the inner membrane transporters Tim23, Tim17 and Tim22.Citation7 shows the locations of PRAT family members (indicated in bold), mitochondrial protein import components (indicated in bold) and Complex I subunits within protein complexes of the Arabidopsis mitochondrial proteome as determined by Gelmap (http://www.gelmap.de), which provides a comprehensive annotation of the mitochondrial protein complex proteome from ArabidopsisCitation9,Citation10 and from studies in our laboratory (Wang et al., 2012). In addition to the presence of Tim23–2 and B14.7 in both Complex I and TIM17:23, several Complex I subunits encoded by At1g68680, At2g27730, At4g34700 and At1g72170 also migrate with TIM17:23 complex at 160 and 110 kilodaltons (kD) (). Functional characterization of these proteins could reveal a direct role in maintaining a Tim23–2 and Complex I interaction.
Table 1. The localization of import components within respiratory complexes in plant mitochondria. The locations of import components, Preprotein and Amino acid Transporters (PRAT) and Complex I subunits in the Arabidopsis mitochondrial protein complex proteome, as shown in Klodmann et al., 2011 and Wang et al., 2012. The PRAT components and import components are indicated in bold
Tim22, another PRAT family member, responsible for the translocation of mitochondrial carrier type proteins into the inner membrane was also identified to interact with both Complex I+III and Complex I alone by Klodmann et al., 2011 (). In yeast, Tim22 was also shown to interact with Complex II, via subunit 3 of succinate dehydrogenase (Sdh3).Citation11 Sdh3 was shown to have a dual role, both in electron transfer and in protein import.Citation11 Sdh3 was identified to be both a component of the TIM22 complex and the SDH complex II.Citation11 Another extensively documented example of an interaction between respiratory chain subunits and proteins of the mitochondrial import apparatus is seen with the Mitochondrial Processing Peptidase (MPP). The α and β subunits of MPP are components of the cytochrome bc1 complex (Complex III), a situation that is unique to plant mitochondria.Citation12-Citation15 As expected, MPPα and MPPβ, migrate in BN-PAGE gels with Complex III and also TIM17:23 ().Citation9 MPP is responsible for the removal of the presequences during or after translocation of the preprotein through the TIM17:23 channel, prior to correct sorting and assembly. This dual role of MPP in respiration and protein import was proposed as a mechanism to link mitochondrial activity and biogenesis over a decade agoCitation14 even though the translocation channel and processing ability could not be linked in vitro.Citation16 The physical interactions of TIM17:23 with respiratory Complex III is consistent with this hypothesis, and not only would this allow the efficiency of import be optimized, but the removal of the targeting presequence, which a prerequisite for further processing or assembly, would also take place close to the site of protein translocation.
Coordination of mitochondrial biogenesis and function
While several examples have been documented showing an interaction of the respiratory chain and the protein import apparatus, the functional significance of these interactions is unclear. However, a recent examination of Arabidopsis mutant lines has provided some insight into the biological significance of these interactions.Citation1 Characterization of a Tim23–2 overexpressing line (Tim23–2 OE) found that it exhibited a severe reduction in the abundance of Complex I.Citation1 In agreement, several Complex I T-DNA insertional knockout lines exhibited an induction in Tim23–2 1. The abundance of Tim23–2 and the reduction of Complex I appeared to be interlinked and all mutants exhibited similar retarded growth phenotypes. Not surprisingly, the Tim23–2 OE line and the Complex I knockout plants exhibited an increase in import ability and an induction of several other mitochondrial import components. Transcript analysis of Tim23–2 OE plants also showed a general upregulation of components associated with mitochondrial biogenesis, ranging from components associated with the transcription and translation of mitochondrial located genes to several components of the mitochondrial respiratory electron transport chain.Citation1 Transcript abundance of mitochondrial located genes displayed a 4–10 fold increase in abundance, in organello translation assays also showed that in the Tim23–2 OE line, the rate of translation was increased compared with wild-type (Col-0) plants.Citation1 These data showed that the overexpression of Tim23–2 resulted in the instability of respiratory Complex I. The result was an increase in mitochondrial biogenesis, which included increased import ability, mitochondrial transcription and translation, and a widespread increase in the transcript abundance for a variety of genes encoding mitochondrial components.Citation1 A model for the interactions between Tim23 and Complex I is proposed in . The model proposes that the interaction of Tim23 with Complex I is a molecular mechanism to regulate both mitochondrial biogenesis and respiration. Complex I and TIM17:23 complexes are shown as a “static” model based on the identification of subunits associated with these complexes from a variety of studies. A dynamic model is presented that is proposed to represent the in vivo interactions, showing TIM17:23 interacting with Complex III via Tim21, and TIM17:23 interacting with Complex I, via B14.7. The overexpression of Tim23–2 results in an increase in the amount of TIM17:23sort, as it was observed that Tim50 and Tim21 also increased in the Tim23–2 OE line.Citation1 An increase in the amount of TIM17:23sort results in the instability of Complex I or an inability to assemble Complex I, due to the interactions of Tim23–2 with Complex I subunits, most likely via B14.7. An analysis of the import of radiolabelled Complex I subunits in the Tim23–2 OE lines suggests an inability to assemble complex I, while assembly of subunits into other complexes is not affected.Citation1
Figure 1. Diagrammatic representation of the interaction between Tim23–2 and Complex I in Arabidopsis mitochondria. Top left panel – Static – represents the TOM, TIM and respiratory complexes I and III as defined by biochemical and genetic studies. The bottom left panel – Dynamic- represents the interactions between the complexes, and Tim21 and Tim23. Imported Tim23 is incorporated into Complex I, and imported Tim21 is incorporated into Complex III. However, as most of the Tim23 and Tim21 proteins are present in the TIM17:23 complex, and the respiratory complexes are more abundant than TIM17:23 complexes, the association of Tim23 and Tim21 with Complex I and Complex III is not in stoichiometric levels, indicated in blue. A supercomplex of complex I+III is indicated, which does not contain Tim23, Tim21 or the Complex I subunit B14.7. The right panel shows what happens when an increase in Tim23–2 or decrease in Complex I occurs. There is an increase in the Tom20 receptors proteins and proteins of the TIM17:23sort complex, indicated in red. Monomeric Complex I is almost completely absent, as indicated by open blue symbols, and the super-complex I+III is reduced in abundance, indicated in blue. In addition, there are global changes in the cell transcriptome, which includes an increase in mitochondrial transcription and translation, constituting mitochondrial biogenesis.
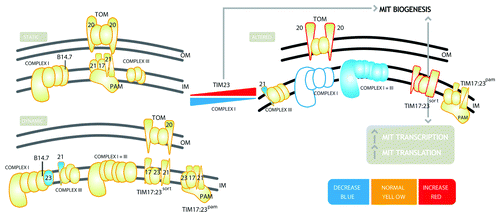
Therefore, in addition to being a subunit of the TIM17:23 translocase, the amount of Tim23–2 in Arabidopsis is finely regulated with Complex I, any decrease in Complex I results in an increase in TIM17:23 and vice versa. This provides for an elegant system for retrograde signaling to control the expression of nuclear encoded genes required for mitochondrial biogenesis and respiratory chain activity. A decrease in the amount of Complex I, due to oxidative damage or an increased respiratory demand, which may change the amount of monomeric Complex I, via interaction with Complex III to form a supercomplex of I+III, will result in signals that increase the amount of Tim23–2 and thus activate mitochondrial biogenesis. This model provides some functional insight into one of the possible reasons for why the mitochondrial preprotein transporters and respiratory complexes are linked.
It should also be noted that in both bovine and Neurospora crassa, Complex I contains a B14.7 type PRAT subunit,Citation17,Citation18 thus a similar situation may occur in these organisms. Notably, yeast is unusual as it lacks Complex I, and thus the model system normally used to study mitochondrial protein import (yeast), cannot provide insight into this regulatory loop, where mitochondrial activity can signal mitochondrial biogenesis via retrograde regulatory pathways. The reason this may differ in yeast, is that as a facultative anaerobe, mitochondrial biogenesis and mitochondrial respiratory chain activity need to be independent and uncoupled. Thus, in yeast, the genes encoding respiratory chain proteins are regulated by the availability of oxygen, via the synthesis of heme.Citation2 However, in other organisms that are obligatory aerobes, the link between respiratory chain activity and biogenesis provides a means to adjust mitochondrial activity to the requirements of the cell. Finally, in Arabidopsis, the discovery of this novel regulatory loop will allow approaches to identify the factors that are responsible for mitochondrial biogenesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the University of Western Australia Scholarship for International Research Fees grant to Y.W., an Australian Research Council Australian Postdoctoral Fellowship grant to M.W.M., and the Australian Research Council Centre of Excellence Program CEO561495.
References
- Wang Y, Carrie C, Giraud E, Elhafez D, Narsai R, Duncan O, et al. Dual location of the mitochondrial preprotein transporters B14.7 and Tim23-2 in complex I and the TIM17:23 complex in Arabidopsis links mitochondrial activity and biogenesis. Plant Cell 2012; 24:2675 - 95; http://dx.doi.org/10.1105/tpc.112.098731; PMID: 22730406
- Kwast KE, Burke PV, Poyton RO. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J Exp Biol 1998; 201:1177 - 95; PMID: 9510529
- Klodmann J, Braun HP. Proteomic approach to characterize mitochondrial complex I from plants. Phytochemistry 2011; 72:1071 - 80; http://dx.doi.org/10.1016/j.phytochem.2010.11.012; PMID: 21167537
- Meyer EH, Taylor NL, Millar AH. Resolving and identifying protein components of plant mitochondrial respiratory complexes using three dimensions of gel electrophoresis. J Proteome Res 2008; 7:786 - 94; http://dx.doi.org/10.1021/pr700595p; PMID: 18189341
- van der Laan M, Wiedemann N, Mick DU, Guiard B, Rehling P, Pfanner N. A role for Tim21 in membrane-potential-dependent preprotein sorting in mitochondria. Curr Biol 2006; 16:2271 - 6; http://dx.doi.org/10.1016/j.cub.2006.10.025; PMID: 17113393
- Chacinska A, van der Laan M, Mehnert CS, Guiard B, Mick DU, Hutu DP, et al. Distinct forms of mitochondrial TOM-TIM supercomplexes define signal-dependent states of preprotein sorting. Mol Cell Biol 2010; 30:307 - 18; http://dx.doi.org/10.1128/MCB.00749-09; PMID: 19884344
- Murcha MW, Elhafez D, Lister R, Tonti-Filippini J, Baumgartner M, Philippar K, et al. Characterization of the preprotein and amino acid transporter gene family in Arabidopsis. Plant Physiol 2007; 143:199 - 212; http://dx.doi.org/10.1104/pp.106.090688; PMID: 17098851
- Rassow J, Dekker PJ, van Wilpe S, Meijer M, Soll J. The preprotein translocase of the mitochondrial inner membrane: function and evolution. J Mol Biol 1999; 286:105 - 20; http://dx.doi.org/10.1006/jmbi.1998.2455; PMID: 9931253
- Klodmann J, Senkler M, Rode C, Braun HP. Defining the protein complex proteome of plant mitochondria. Plant Physiol 2011; 157:587 - 98; http://dx.doi.org/10.1104/pp.111.182352; PMID: 21841088
- Rode C, Senkler M, Klodmann J, Winkelmann T, Braun HP. GelMap-a novel software tool for building and presenting proteome reference maps. J Proteomics 2011; 74:2214 - 9; http://dx.doi.org/10.1016/j.jprot.2011.06.017; PMID: 21729775
- Gebert N, Gebert M, Oeljeklaus S, von der Malsburg K, Stroud DA, Kulawiak B, et al. Dual function of Sdh3 in the respiratory chain and TIM22 protein translocase of the mitochondrial inner membrane. Mol Cell 2011; 44:811 - 8; http://dx.doi.org/10.1016/j.molcel.2011.09.025; PMID: 22152483
- Brumme S, Kruft V, Schmitz UK, Braun HP. New insights into the co-evolution of cytochrome c reductase and the mitochondrial processing peptidase. J Biol Chem 1998; 273:13143 - 9; http://dx.doi.org/10.1074/jbc.273.21.13143; PMID: 9582354
- Eriksson AC, Sjöling S, Glaser E. Characterization of the bifunctional mitochondrial processing peptidase (MPP)/bc1 complex in Spinacia oleracea. J Bioenerg Biomembr 1996; 28:285 - 92; http://dx.doi.org/10.1007/BF02110702; PMID: 8807403
- Glaser E, Dessi P. Integration of the mitochondrial-processing peptidase into the cytochrome bc1 complex in plants. J Bioenerg Biomembr 1999; 31:259 - 74; http://dx.doi.org/10.1023/A:1005475930477; PMID: 10591532
- Glaser E, Eriksson A, Sjöling S. Bifunctional role of the bc1 complex in plants. Mitochondrial bc1 complex catalyses both electron transport and protein processing. FEBS Lett 1994; 346:83 - 7; http://dx.doi.org/10.1016/0014-5793(94)00312-2; PMID: 8206164
- Dessi P, Rudhe C, Glaser E. Studies on the topology of the protein import channel in relation to the plant mitochondrial processing peptidase integrated into the cytochrome bc1 complex. Plant J 2000; 24:637 - 44; http://dx.doi.org/10.1046/j.1365-313x.2000.00910.x; PMID: 11123802
- Carroll J, Shannon RJ, Fearnley IM, Walker JE, Hirst J. Definition of the nuclear encoded protein composition of bovine heart mitochondrial complex I. Identification of two new subunits. J Biol Chem 2002; 277:50311 - 7; http://dx.doi.org/10.1074/jbc.M209166200; PMID: 12381726
- Nehls U, Hemmer S, Röhlen DA, Van der Pas JC, Preis D, Sackmann U, et al. cDNA and genomic DNA sequence of the 21.3 kDa subunit of NADH:ubiquinone reductase (complex I) from Neurospora crassa. Biochim Biophys Acta 1991; 1088:325 - 6; http://dx.doi.org/10.1016/0167-4781(91)90074-V; PMID: 1825789