Abstract
Recent research findings clearly indicate that lysin motif (LysM)-containing cell surface receptors are involved in the recognition of specific oligosaccharide elicitors (chitin and peptidoglycan), which trigger an innate immunity response in plants. These receptors are either LysM-containing receptor-like kinases (LYKs) or LysM-containing receptor proteins (LYPs). In Arabidopsis, five LYKs (AtCERK1/AtLYK1 and AtLYK2–5) and three LYPs (AtLYP1–3) are likely expressed on the plasma membrane. In this review, we summarize recent research results on the role of these receptors in plant innate immunity, including the recent structural characterization of AtCERK1 and composition of the various receptor complexes in Arabidopsis.
Multicellular organisms activate immune systems upon recognition of microbe-derived non-self components, known as microbe elicitors or microbe-associated molecular patterns (MAMPs; synonymously termed pathogen-associated molecular patterns, PAMPs), which are invariant structures originating from microbial components and not present in the host. MAMPs are usually recognized by pattern recognition receptors (PRRs) on the cell surface triggering plant innate immunity responses.Citation1-Citation3
Oligosaccharide MAMPs, mostly microbial cell envelope components, are represented by bacterial lipopolysaccharide (LPS), peptidoglycan (PGN) and fungal chitin.Citation4 LPS is an outer membrane glycoconjugate from Gram-negative bacteria that is composed of a lipid and a polysaccharide joined by a covalent bond. Plant cells likely sense the sugar and lipid components of LPS separately.Citation5 However, the plant LPS receptor(s) has not been identified. PGN is an essential cell wall component in both Gram-positive and Gram-negative bacteria. The structure of PGN is similar to chitin being composed of alternating residues of β-1,4-linked N-acetyl-glucosamine (GlcNAc) and N-acetylmuramic acid, with a short peptide chain attached. PGNs from both Gram-positive and Gram-negative bacteria can elicit defense responses in plants.Citation6-Citation8 Chitin is a homopolymer of β-1,4-linked GlcNAc (chitooligosaccharides), the major structural component of fungal cell walls, and is a potent elicitor on plants.Citation9,Citation10 During plant-pathogen interaction, the fungal cell wall is degraded by chitinases releasing the chitooligosaccharide MAMP elicitors.Citation4 The PRRs for both PGN and chitin have been identified as lysin motif (LysM)-containing proteins as described below.
The first chitin receptor was identified in rice, the chitin elicitor-binding protein (CEBiP), encoding a LysM receptor protein, which lacks an intracellular kinase domain.Citation11 Subsequently, in Arabidopsis, the primary chitin receptor was identified as the chitin elicitor receptor kinase 1 (CERK1), which encodes a protein with extracellular LysM-domains, a transmembrane domain and an active, intracellular kinase domain.Citation12,Citation13 Both in rice and in Arabidopsis, these respective receptors were found to be essential for chitin-triggered innate immunity. For example, mutants in these receptors are compromised in their defense against fungal pathogens, indicating that perception of chitin fragments plays a critical role in pathogen resistance.Citation11-Citation13 All the chitin and PGN receptors identified to date contain one or more extracellular LysM domains.Citation14,Citation15 The LysM domain was first identified in bacterial enzymes involved in remodeling peptidoglycan structure.Citation16-Citation18 Consistent with a functional role in binding PGN, LysM-containing proteins (LYPs) were identified as plant PGN receptors that directly bind to PGNs.Citation19,Citation20 Mutations in these PGN receptor genes completely blocked the plant response to PGN elicitation.
AtCERK1/AtLYK1 is a Major Chitin Receptor, Which is Also Required for PGN Recognition
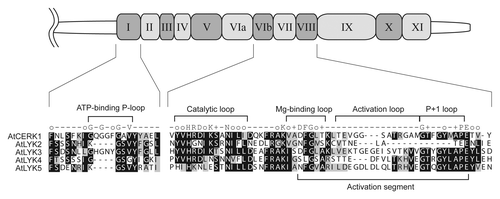
In Arabidopsis, the cell surface receptor AtCERK1 (also termed LysM-containing receptor-like kinase1, LYK1) is an essential PRR for sensing fungal-derived chitooligosaccharides and for immunity to fungal infection.Citation12,Citation13 AtCERK1 binds chitooligosaccharides by its extracellular LysM domains and presumably initiates intracellular signal transduction through activation of its cytoplasmic protein kinase domain.Citation21,Citation22 Interestingly, the AtCERK1 receptor is the first PRR that lacks a non-arginine-aspartate (non-RD) signaling domain; it has a typical RD signaling domain in its catalytic loop () and possesses autophosphorylation activity.Citation22
Figure 1. Sequence comparison of the kinase domain of Arabidopsis LYK proteins. Schematic representation shows the kinase domain of AtCERK1 (drawn to scale). Kinase subdomain I and VIb to VIII are selectively highlighted below the drawing to highlight the five AtLYK proteins. Identical and similar residues throughout the alignment are shown in black and gray, respectively. The consensus line among the eukaryotic protein kinase superfamilyCitation47 is given according to the following code: uppercase letters, invariant residues; o, conserved nonpolar residues; +, conserved small residues with near neutral polarity. Note that AtCERK1 and AtLYK3 have an intact intracellular kinase domain.
In rice, Arabidopsis, and most plants, maximal activation of innate immunity requires longer-chain chitin oligomers [degree of polymerization (dp) = 7–8 GlcNAc residues].Citation22-Citation24 However, shorter-chain oligomers (dp < 6) do bind to AtCERK1.Citation21,Citation22 Very recently, Liu et al.Citation25 elucidated the X-ray crystal structure of the extracellular domain of AtCERK1, as well as characterized the chitooligosaccharide-binding activity of this protein using a variety of methods (isothermal calorimetric analysis, etc.). These results indicate that the chitotetraose binds exclusively to the second LysM domain, bracketed by the two additional LysM domains found in the AtCERK1 protein. The binding affinities of AtCERK1 for chitin oligomers were found to be in the low µM range, which is inconsistent with the very high affinities (nM range) suggested by physiological experiments that measure the plant response to chitin elicitation. Also inconsistent with physiological experiments, the affinity for short-chain chitin oligomers (dp = 5) was roughly similar to that of the long-chain oligomers (dp = 8). Currently, there is no explanation for the differences seen in the affinity of chitooligosaccharide binding to the purified AtCERK1 and the apparent high affinity suggested by measuring the plant response to chitin elicitation.
The structural studies do provide an explanation as to why only the longer chain oligomers are strong inducers of innate immunity. Liu et al.Citation25 showed that, while AtCERK1 binds to the short-chain chitin oligomers, only the long-chain chitin oligomers (dp = 7 or 8) induce homodimerization of the receptor, which was shown to be essential for activation of downstream signaling. In contrast to the situation in Arabidopsis, the functional role of OsCERK1 appears to be quite different. In rice, OsCERK1 does not bind to chitooligosaccharides. Instead, the co-receptor, OsCEBiP is essential for chitin binding, which leads to heterodimerization of the receptor complex and subsequently activation of innate immunity.Citation26 Unlike AtCERK1 that contains three LysM domains, the OsCERK1 contains only one conserved LysM domain.Citation26 Given the fact that the requirement of three LysM domains is essential for chitin binding,Citation22 the differences in the chitin perception systems between rice and Arabidopsis may be explained by the structural differences between AtCERK1 and OsCERK1, although these two proteins do appear to be orthologous based on sequence comparisons.Citation26 The rice model may also apply to other grass speciesCitation27 since, for example, a barley homolog of OsCEBiP, HvCEBiP, was recently shown to contribute to fungal resistance.Citation28
The rice chitin receptor complex, being composed of OsCERK1 and OsCEBiP, is more similar to the proposed PGN receptor complex in Arabidopsis. In this latter case, PGN recognition requires two, non-redundant LYPs, AtLYP2/LYM1 and AtLYP3/LYM3, but also AtCERK1.Citation20 However, direct interaction between these proteins has yet to be shown experimentally. The rice PGN receptor complex is likely similar to that in Arabidopsis since recent work showed that OsLYP4 and OsLYP6, the closest homologs of AtLYP2 and AtLYP3 in rice, are essential for perception of PGN and chitin.Citation19 OsLYP4 and OsLYP6 are distinct proteins from OsCEBiP (OsLYP1). A role of OsCERK1 in PGN recognition in rice has not been reported.
Five AtLYK and Three AtLYP Proteins
Published data clearly show that AtCERK1 is the major chitin receptor in Arabidopsis and essential for the induction of innate immunity upon chitin elicitation. However, Arabidopsis also has other LysM receptor proteins and, therefore, what is their function, relative to chitin or PGN recognition and innate immunity? We here designate the Arabidopsis LYKs as AtCERK1/AtLYK1 and AtLYK2–5, and LYPs as AtLYP1–3 to avoid any confusion ( shows a summary); a nomenclature based on our earlier publication.Citation14,Citation15
Table 1. LysM-containing receptors in Arabidopsis
As mentioned above, AtLYP2 and AtLYP3 are involved in PGN recognition and play indispensable roles for immunity to bacterial infection. Two independent studies demonstrated that neither of these proteins, in contrast to the rice LYPs, binds to chitin oligosaccharides.Citation20,Citation29 Therefore, AtLYP2 and AtLYP3 appear to specifically respond to bacterial PGN elicitors.
Three different labs reported that, in addition to AtCERK1, other LysM-containing receptors, AtLYK4, AtLYK5 and AtLYP1 are able to bind to chitin molecules.Citation22,Citation29,Citation30 AtLYP1, an OsCEBiP homolog, showed high-affinity binding as measured using biotinylated chitooligosaccharides.Citation29 The binding characteristics of AtLYP1 were very similar to that of OsCEBiP. However, mutants disrupted in the expression of AtLYP1, AtLYP2 and AtLYP3, either singly or in combination, showed no difference from the wild-type in their response to chitin elicitation.Citation29,Citation30 Therefore, it would appear that none of the Arabidopsis OsCEBiP homologs are necessary for chitin recognition. Similar mutant studies demonstrated that AtLYK2, AtLYK3 and AtLYK5 do not appear to be involved in chitin recognition.Citation30
AtLYK4 is Important for Chitin-Mediated Innate Immunity
Wan et al.Citation30 recently reported that a knockout mutation of AtLYK4 reduced, but did not eliminate, the response to chitin elicitation, e.g., induction of chitin-responsive gene expression and elevation of cytosolic calcium levels. In contrast, a knockout mutant of AtCERK1 shows essentially no response to chitin elicitation. The data suggest that, while not essential for the chitin response, AtLYK4 is involved in chitin recognition, perhaps as a co-receptor to increase AtCERK1 affinity or activity. As would be expected, AtLYK4 mutant plants showed enhanced susceptibility to both the fungal pathogen Alternaria brassicicola and the bacterial pathogen Pseudomonas syringae pv tomato DC3000. These data raise the possibility that AtLYK4 may also be involved in responding to bacterially produced MAMPs (e.g., PGN). However, this hypothesis remains to be tested.
An analysis of Arabidopsis plants expressing a AtLYK4 promoter-GUS construct showed that this gene is expressed in most tissues (except for flowers, pollens and siliques).Citation30 Interestingly, in leaves, AtLYK4 is predominantly expressed in hydathodes.Citation30 Hydathodes are open pores located on the margin of leaves, which permit the discharge of excess water from the plant, but lack structural barriers against pathogens. A number of pathogens likely enter leaves through hydathodes in addition to leaf stomatal openings.Citation31 In fact, chitinases and other genes related to the defense response are highly expressing in hydathodes, presumably to position them to respond to the invading pathogens.Citation32,Citation33 The restricted expression of the AtLYK4 gene in the hydathodes contrasts with that of AtCERK1, which is expressed throughout the leaf tissue. This difference suggests that the rather moderate phenotype of lyk4 mutant plants, relatively to cerk1 mutants, could be due to the restricted expression of AtLYK4. If true, this might suggest that other LysM receptor proteins could substitute for the role of AtLYK4 depending on their specific pattern of tissue expression. Such functional redundancy among LysM receptors would also explain why mutations in the various receptor genes result in no observable phenotypic changes in the plants relative to the chitin response.
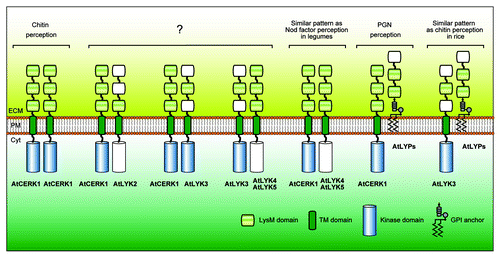
Unlike AtCERK1, in vitro assays failed to demonstrate kinase activity in purified preparations of AtLYK4.Citation30 These results are supported by an analysis of the AtLYK4 protein sequence that shows the lack of critical residues necessary for kinase catalytic activity (). For example, AtLYK4 differs significantly from AtCERK1 in the ATP-binding P-loop (GxGxF/YG) and Mg-binding loop (DFG). If AtCERK1 and AtLYK4 do indeed form a receptor complex then this would resemble the receptor complex involved in recognition of lipo-chitooligosaccharides in the legume Lotus japonicus.Citation34 Recognition of the lipo-chitooligosaccharide Nod factor, produced by the legume bacterial symbiont, is essential for formation of the nitrogen-fixing symbiosis. The Nod factor receptor complex is composed of LjNFR1, an active kinase homolog of AtCERK1, and LjNFR5, an inactive kinase homolog of AtLYK4. Therefore, LjNFR5 and AtLYK4 appear to be (lipo-) chitooligosaccharide-binding receptor-like kinases with a pseudo kinase domain (categorized as LysM-RLK II or LYR; see ).Citation35,Citation36 While genetic data suggest that AtLYK4 may partner with AtCERK1 to compose the chitin receptor, another possible partner could be AtLYK3, which also possesses a functional, intracellular kinase domain ( and ). However, AtLYK3 has no known function since mutations do not appear to affect plant susceptibility to either fungal or bacterial pathogens.Citation30
Figure 3. Possible combinations of Arabidopsis LysM receptor proteins involved in MAMP recognition. The picture represents theoretical combinations of LysM receptors with a prerequisite condition that two or more LysM domains are present in the extracellular region and that at least one of the receptors possess an active, intracellular kinase domain. Putative LysM domains with less sequence conservation and pseudo kinase domains are drawn colorless. Note that AtCERK1 is a unique LysM receptor in Arabidopsis since it alone can form a homodimer given the prerequisites imposed. Abbreviations: LysM, lysin motif; TM, transmembrane; GPI, glycosylphosphatidylinositol; ECM, extracellular matrix; PM, plasma membrane; Cyt, cytoplasm.
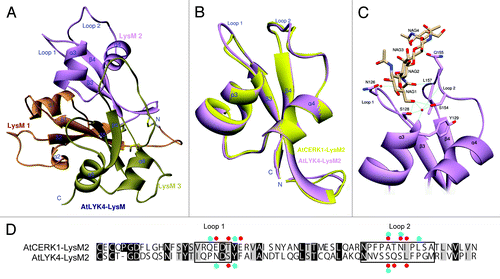
As mentioned above, independent laboratories showed that AtLYK4 can bind to chitin.Citation22,Citation29,Citation30 Therefore, utilizing the published X-ray crystal structure of AtCERK1, we modeled the structure of the AtLYK4 extracellular domain (). The computer modeling was performed as described previously.Citation37 This model revealed that AtLYK4 uses fewer and different amino acid residues than AtCERK1 to interact with chitotetraose at the second LysM domain (), suggesting that AtLYK4 likely has lower binding affinity to chitotetraose than AtCERK1. Indeed, the AutoDock program calculates a binding affinity of -8.6 kcal/mol for AtLYK4 and -9.2 kcal/mol for AtCERK1, respectively. However, the predicted model shows that the second LysM domain has a very similar structure between AtLYK4 and AtCERK1 (the root-mean-square deviation values are 0.648 Å and 0.893 Å for whole extracellular domain, respectively) (). This prediction of a lower affinity for AtLYK4 is consistent with the previous reports that this protein showed a weaker interaction with a chitin bead column than AtCERK1.Citation22,Citation30
Figure 2. Three-dimensional model prediction of the structure of the AtLYK4 extracellular domain, including a ligand docking model. (A)The 3D model of AtLYK4 extracellular domain was built based on the crystal structure of AtCERK1 (PDB code: 4EBY). Each LysM domain is represented in a different color: first LysM (orange), second LysM (purple) and third LysM (green). (B)The second LysM domains of AtLYK4 (purple) and AtCERK1 (yellow) are superimposed to highlight the similarity in structure. Note that the overall folds are highly conserved between the two models, although the AtLYK4 has the longer extended Loop 1 which is a constitutive part of the cleft where the predicted chitin-binding site is found. (C) Docking model between the second LysM domain of AtLYK4 and chitotetraose. (D) Pairwise sequence comparison of the second LysM domains of AtLYK4 and AtCERK1. Identical and similar residues throughout the alignment are shown in black and gray, respectively. Enclosed boxes represent Loop 1 and Loop 2. Red and blue dots indicate the residues involved in direct interactions and water molecule-mediated interactions with chitotetraose, respectively. The residues involved in van der Waals interactions were neglected here.
Perspectives
Recent research findings have provided a significant amount of additional detail regarding the role of LysM receptors (especially AtCERK1, AtLYK4, AtLYP2 and AtLYP3) in chitin and PGN recognition, as well as their role in plant innate immunity. However, there are still many unanswered questions regarding the exact composition of the respective receptor complexes, other auxiliary proteins, the mechanism of signaling and other components of the signaling cascade leading ultimately to enhanced disease resistance.
Although it is clear that some LysM receptor proteins have affinity for chitin-like molecules, the biological/biochemical function of the other Arabidopsis LysM receptor proteins remains enigmatic (i.e., AtLYK2, AtLYK3, AtLYK5 and AtLYP1). In these cases, mutant studies have not suggested a functional role for these proteins. However, sequence analysis and biochemical assays indicate that AtLYK5 and AtLYP1 likely recognize chitin molecules and AtLYK3 likely possesses a functional, intracellular kinase domain. All of these proteins are presumably located in the plasma membrane due to the presence of an N-terminal signal peptide and a transmembrane segment or a GPI anchor signal sequences for membrane attachment ( and ). However, this prediction needs to be confirmed by experimentation. Unlike AtCERK1, AtLYK2 and AtLYK3 have only one conserved LysM domain in their extracellular domain ( and ). Whether this would disrupt interaction with an extracellular ligand is not clear. AtLYK2 and AtLYK5 likely lack an active, intracellular kinase domain (), suggesting that they may function in conjunction with a co-receptor. It may be that one or more of these proteins do function in MAMP signaling, which is being masked in mutant studies due to functional redundancy. It is still possible that these receptors are functioning in a spatial or temporal manner,Citation38 which would make the identification of a mutant phenotype difficult. It is also possible that one or more of these LysM-domain proteins could bind MAMPs but not induce downstream signaling, for example, as a decoy receptor. Such an example has been found for plant recognition of the fungal elicitor xylanase where only one receptor (Eix2) mediates defense signaling while the other (Eix1) acts as a decoy receptor to attenuate the MAMP response.Citation39,Citation40 Decoy receptors are well-known in mammals,Citation41-Citation43 in which a decoy receptor manipulates the signaling of a cognate receptor by competing for ligand binding resulting in inhibition of downstream signaling to regulate tumor proliferation and cell death. If acting as a decoy, the LysM receptor protein could attenuate the MAMP response by titrating the elicitor in the extracellular matrix. Alternatively, it is still possible that these LYKs or LYPs recognize different size chitin oligomers, since it is possible that different oligomers could have distinct functions,Citation44,Citation45 as shown for chitin recognition by mammalian macrophages.Citation46 Further studies are required to disclose the nature of molecular functions of all LysM receptors in plant innate immunity.
Acknowledgments
This work was supported by a grant from the Office of Basic Energy Sciences, the United States Department of Energy (grant no. DE-FG02–08ER15309) and by the Next-Generation BioGreen 21 Program Systems and Synthetic Agrobiotech Center, Rural Development Administration, Republic of Korea (grant no. PJ009068).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 2006; 124:803 - 14; http://dx.doi.org/10.1016/j.cell.2006.02.008; PMID: 16497589
- Janeway CA Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20:197 - 216; http://dx.doi.org/10.1146/annurev.immunol.20.083001.084359; PMID: 11861602
- Jones JD, Dangl JL. The plant immune system. Nature 2006; 444:323 - 9; http://dx.doi.org/10.1038/nature05286; PMID: 17108957
- Silipo A, Erbs G, Shinya T, Dow JM, Parrilli M, Lanzetta R, et al. Glyco-conjugates as elicitors or suppressors of plant innate immunity. Glycobiology 2010; 20:406 - 19; http://dx.doi.org/10.1093/glycob/cwp201; PMID: 20018942
- Silipo A, Molinaro A, Sturiale L, Dow JM, Erbs G, Lanzetta R, et al. The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris.. J Biol Chem 2005; 280:33660 - 8; http://dx.doi.org/10.1074/jbc.M506254200; PMID: 16048996
- Erbs G, Silipo A, Aslam S, De Castro C, Liparoti V, Flagiello A, et al. Peptidoglycan and muropeptides from pathogens Agrobacterium and Xanthomonas elicit plant innate immunity: structure and activity. Chem Biol 2008; 15:438 - 48; http://dx.doi.org/10.1016/j.chembiol.2008.03.017; PMID: 18482696
- Gust AA, Biswas R, Lenz HD, Rauhut T, Ranf S, Kemmerling B, et al. Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J Biol Chem 2007; 282:32338 - 48; http://dx.doi.org/10.1074/jbc.M704886200; PMID: 17761682
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, et al. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 2010; 22:973 - 90; http://dx.doi.org/10.1105/tpc.109.069658; PMID: 20348432
- Felix G, Regenass M, Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J 1993; 4:307 - 16; http://dx.doi.org/10.1046/j.1365-313X.1993.04020307.x
- Lee CG, Da Silva CA, Lee JY, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr Opin Immunol 2008; 20:684 - 9; http://dx.doi.org/10.1016/j.coi.2008.10.002; PMID: 18938241
- Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA 2006; 103:11086 - 91; http://dx.doi.org/10.1073/pnas.0508882103; PMID: 16829581
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 2007; 104:19613 - 8; http://dx.doi.org/10.1073/pnas.0705147104; PMID: 18042724
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 2008; 20:471 - 81; http://dx.doi.org/10.1105/tpc.107.056754; PMID: 18263776
- Zhang XC, Cannon SB, Stacey G. Evolutionary genomics of LysM genes in land plants. BMC Evol Biol 2009; 9:183; http://dx.doi.org/10.1186/1471-2148-9-183; PMID: 19650916
- Zhang XC, Wu X, Findley S, Wan J, Libault M, Nguyen HT, et al. Molecular evolution of lysin motif-type receptor-like kinases in plants. Plant Physiol 2007; 144:623 - 36; http://dx.doi.org/10.1104/pp.107.097097; PMID: 17449649
- Buist G, Steen A, Kok J, Kuipers OP. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol 2008; 68:838 - 47; http://dx.doi.org/10.1111/j.1365-2958.2008.06211.x; PMID: 18430080
- Joris B, Englebert S, Chu CP, Kariyama R, Daneo-Moore L, Shockman GD, et al. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol Lett 1992; 70:257 - 64; http://dx.doi.org/10.1111/j.1574-6968.1992.tb05218.x; PMID: 1352512
- Steen A, Buist G, Leenhouts KJ, El Khattabi M, Grijpstra F, Zomer AL, et al. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J Biol Chem 2003; 278:23874 - 81; http://dx.doi.org/10.1074/jbc.M211055200; PMID: 12684515
- Liu B, Li JF, Ao Y, Qu J, Li Z, Su J, et al. Lysin motif-containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell 2012; 24:3406 - 19; http://dx.doi.org/10.1105/tpc.112.102475; PMID: 22872757
- Willmann R, Lajunen HM, Erbs G, Newman MA, Kolb D, Tsuda K, et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc Natl Acad Sci USA 2011; 108:19824 - 9; http://dx.doi.org/10.1073/pnas.1112862108; PMID: 22106285
- Iizasa E, Mitsutomi M, Nagano Y. Direct binding of a plant LysM receptor-like kinase, LysM RLK1/CERK1, to chitin in vitro. J Biol Chem 2010; 285:2996 - 3004; http://dx.doi.org/10.1074/jbc.M109.027540; PMID: 19951949
- Petutschnig EK, Jones AM, Serazetdinova L, Lipka U, Lipka V. The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J Biol Chem 2010; 285:28902 - 11; http://dx.doi.org/10.1074/jbc.M110.116657; PMID: 20610395
- Shibuya N, Minami E. Oligosaccharide signalling for defence responses in plant. Physiol Mol Plant Pathol 2001; 59:223 - 33; http://dx.doi.org/10.1006/pmpp.2001.0364
- Wan J, Zhang S, Stacey G. Activation of a mitogen-activated protein kinase pathway in Arabidopsis by chitin. Mol Plant Pathol 2004; 5:125 - 35; http://dx.doi.org/10.1111/j.1364-3703.2004.00215.x; PMID: 20565589
- Liu T, Liu Z, Song C, Hu Y, Han Z, She J, et al. Chitin-induced dimerization activates a plant immune receptor. Science 2012; 336:1160 - 4; http://dx.doi.org/10.1126/science.1218867; PMID: 22654057
- Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J 2010; 64:204 - 14; http://dx.doi.org/10.1111/j.1365-313X.2010.04324.x; PMID: 21070404
- Balmer D, Planchamp C, Mauch-Mani B. On the move: Induced resistance in monocots. J Exp Bot 2012; In press http://dx.doi.org/10.1093/jxb/ers248; PMID: 23028020
- Tanaka S, Ichikawa A, Yamada K, Tsuji G, Nishiuchi T, Mori M, et al. HvCEBiP, a gene homologous to rice chitin receptor CEBiP, contributes to basal resistance of barley to Magnaporthe oryzae.. BMC Plant Biol 2010; 10:288; http://dx.doi.org/10.1186/1471-2229-10-288; PMID: 21190588
- Shinya T, Motoyama N, Ikeda A, Wada M, Kamiya K, Hayafune M, et al. Functional characterization of CEBiP and CERK1 homologs in Arabidopsis and rice reveals the presence of different chitin receptor systems in plants. Plant Cell Physiol 2012; 53:1696 - 706; http://dx.doi.org/10.1093/pcp/pcs113; PMID: 22891159
- Wan J, Tanaka K, Zhang XC, Son GH, Brechenmacher L, Nguyen TH, et al. LYK4, a lysin motif receptor-like kinase, is important for chitin signaling and plant innate immunity in Arabidopsis. Plant Physiol 2012; 160:396 - 406; http://dx.doi.org/10.1104/pp.112.201699; PMID: 22744984
- Hugouvieux V, Barber CE, Daniels MJ. Entry of Xanthomonas campestris pv. campestris into hydathodes of Arabidopsis thaliana leaves: a system for studying early infection events in bacterial pathogenesis. Mol Plant Microbe Interact 1998; 11:537 - 43; http://dx.doi.org/10.1094/MPMI.1998.11.6.537; PMID: 9612952
- Kobae Y, Sekino T, Yoshioka H, Nakagawa T, Martinoia E, Maeshima M. Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol 2006; 47:309 - 18; http://dx.doi.org/10.1093/pcp/pcj001; PMID: 16415066
- Samac DA, Shah DM. Developmental and pathogen-induced activation of the Arabidopsis acidic chitinase promoter. Plant Cell 1991; 3:1063 - 72; PMID: 12324582
- Arrighi JF, Barre A, Ben Amor B, Bersoult A, Soriano LC, Mirabella R, et al. The Medicago truncatula lysin [corrected] motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol 2006; 142:265 - 79; http://dx.doi.org/10.1104/pp.106.084657; PMID: 16844829
- Gust AA, Willmann R, Desaki Y, Grabherr HM, Nürnberger T. Plant LysM proteins: modules mediating symbiosis and immunity. Trends Plant Sci 2012; 17:495 - 502; http://dx.doi.org/10.1016/j.tplants.2012.04.003; PMID: 22578284
- Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004; 16:1220 - 34; http://dx.doi.org/10.1105/tpc.020834; PMID: 15105442
- Tanaka K, Nguyen CT, Libault M, Cheng J, Stacey G. Enzymatic activity of the soybean ecto-apyrase GS52 is essential for stimulation of nodulation. Plant Physiol 2011; 155:1988 - 98; http://dx.doi.org/10.1104/pp.110.170910; PMID: 21346172
- Faulkner C, Robatzek S. Plants and pathogens: putting infection strategies and defence mechanisms on the map. Curr Opin Plant Biol 2012; In press http://dx.doi.org/10.1016/j.pbi.2012.08.009; PMID: 22981427
- Bar M, Sharfman M, Avni A. LeEix1 functions as a decoy receptor to attenuate LeEix2 signaling. Plant Signal Behav 2011; 6:455 - 7; http://dx.doi.org/10.4161/psb.6.3.14714; PMID: 21364318
- Bar M, Sharfman M, Ron M, Avni A. BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J 2010; 63:791 - 800; http://dx.doi.org/10.1111/j.1365-313X.2010.04282.x; PMID: 20561260
- Bengtsson AK, Ryan EJ. Immune function of the decoy receptor osteoprotegerin. Crit Rev Immunol 2002; 22:201 - 15; PMID: 12498383
- Mantovani A, Muzio M, Ghezzi P, Colotta F, Introna M. Negative regulators of the interleukin-1 system: receptor antagonists and a decoy receptor. Int J Clin Lab Res 1996; 26:7 - 14; http://dx.doi.org/10.1007/BF02644768; PMID: 8739850
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997; 277:815 - 8; http://dx.doi.org/10.1126/science.277.5327.815; PMID: 9242610
- Khan W, Costa C, Souleimanov A, Prithiviraj B, Smith DL. Response of Arabidopsis thaliana roots to lipo-chitooligosaccharide from Bradyrhizobium japonicum and other chitin-like compounds. Plant Growth Regul 2011; 63:243 - 9; http://dx.doi.org/10.1007/s10725-010-9521-6
- Brotman Y, Landau U, Pnini S, Lisec J, Balazadeh S, Mueller-Roeber B, et al. The LysM receptor-like kinase LysM RLK1 is required to activate defense and abiotic-stress responses induced by overexpression of fungal chitinases in Arabidopsis plants. Mol Plant 2012; 5:1113 - 24; http://dx.doi.org/10.1093/mp/sss021; PMID: 22461667
- Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol 2009; 182:3573 - 82; http://dx.doi.org/10.4049/jimmunol.0802113; PMID: 19265136
- Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 1995; 9:576 - 96; PMID: 7768349