Abstract
Sensing of microbial pathogens by pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs) elicits a defense program known as PAMP-triggered immunity (PTI). Recently, we have shown that the Arabidopsis thaliana L-TYPE LECTIN RECEPTOR KINASE-VI.2 (LecRK-VI.2) positively regulates bacterial PTI. In this report, we suggest by in silico analysis that the kinase domain of LecRK-VI.2 is functional. LecRK-VI.2 also demonstrated auto-phosphorylation activity in vitro in the presence of divalent metal cations indicating that LecRK-VI.2 has the ability to auto-phosphorylate. We further investigate the role of LecRK-VI.2 in Arabidopsis resistance to the necrotrophic fungal pathogen Botrytis cinerea. Disruption of LecRK-VI.2 did not affect Arabidopsis resistance to B. cinerea. Accordingly, wild-type upregulation levels of PTI-responsive WRKY53, FRK1, NHL10, CYP81F2 and CBP60 g after treatment with the fungal PAMP chitin were observed in lecrk-VI.2-1. These data provide evidences that the kinase domain of LecRK-VI.2 is active and show that LecRK-VI.2 is not critical for resistance to the fungal pathogen B. cinerea.
In plants, defense responses are induced by pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs). This recognition activates a defense program known as PAMP-triggered immunity (PTI).Citation1 Arabidopsis defense responses against necrotrophs such as B. cinerea are mediated by jasmonic acid (JA) and/or ethylene (ET) signaling cascades.Citation2 Arabidopsis resistance to B. cinerea is also salicylic acid (SA)-dependent.Citation3,Citation4 Chitin is a major fungal PAMP that triggers the PTI defense response in plants.Citation5 Chitin is produced by B. cinerea and is recognized by CERK1 PRR (chitin elicitor receptor kinase, also known as LysM-RLK1).Citation5 Binding of PAMPs to extracellular domains of receptor-like kinases (RLKs) is thought to activate the intracellular kinase domains of RLKs.Citation6 The lectin receptor kinases are RLKs characterized by the presence of an extracellular legume lectin-like domain, a transmembrane domain and an intracellular serine/threonine (Ser/Thr) kinase (STK) domain. Lectin receptor kinases are divided in three types, G, C, and L based on their extracellular lectin motif.Citation7 Recently, we demonstrated that the L-type lectin receptor kinase VI.2 (LecRK-VI.2) positively regulates Arabidopsis bacteria-mediated PTI.Citation8 Here we suggest that LecRK-VI.2 possesses a functional kinase domain that is able to auto-phosphorylate. In addition, resistance to the necrotrophic fungal pathogen B. cinerea and expression of PTI-responsive genes after chitin treatment were at wild-type levels in a lecrk-VI.2-1 T-DNA insertion mutant. Our results suggest that LecRK-VI.2 specifically regulate bacteria-mediated PTI.
LecRKVI.2 is a Functional Kinase Protein and is not Essential for Basal Resistance to Botrytis cinerea
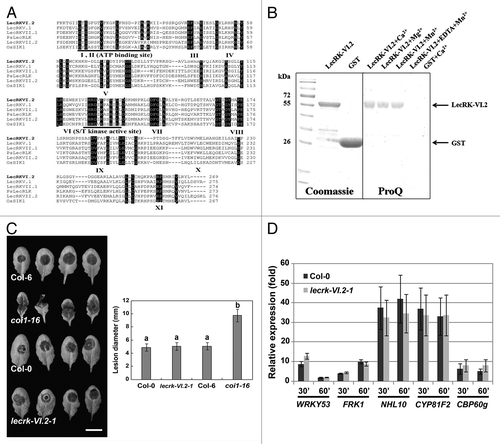
In order to evaluate the functionality of the LecRK-VI.2 kinase domain in silico, amino acid sequences of kinase domain of various published LecRKs such as LecRK-V.1, LecRK-VII.1, and LecRK-VII.2,Citation7 PsLecRLKCitation9 and OsSIK1Citation10 were aligned with the kinase domain (KD) of LecRK-VI.2. We found that the amino acids reported to be essential for catalytic activities of all 11 kinase sub-domainsCitation11 (numbered from I-XI, ) from Arabidopsis, pea and rice were highly conserved in LecRK-VI.2, suggesting that its kinase domain is functional. LecRK-VI.2 exhibited divalent metal cations dependent auto-phosphorylation activity in vitro (). To evaluate Arabidopsis LecRK-VI.2 possible role in the resistance response to other types of pathogens, we inoculated the lecrk-VI.2-1 mutant with the necrotrophic fungus B. cinerea. The mutant lecrk-VI.2-1 demonstrated a wild type resistance response to the necrotroph B. cinerea (). In addition, no significant differences in expression levels of PTI responsive genes between the mutant lecrk-VI.2-1 and the wild-type Col-0 control were observed after chitin treatment (), suggesting that PTI activation after treatment with the fungal PAMP chitin is not dependent on a functional LecRK-VI.2. Together, these observations suggest that LecRK-VI.2 is not critical for Arabidopsis resistance to fungal pathogens.
Figure 1.LecRKVI.2 is a functional protein kinase and is dispensable for Arabidopsis resistance to B. cinerea. (A) Alignment comparison of the predicted amino acid sequences of LecRK-VI.2 with 3 Arabidopsis LecRKs (LecRK-V.1, LecRK-VII.1 and LecRK-VII.2), the rice OsSIK1 and the pea PsLecRLK. Sequences above the Roman numerals in black boxes indicate the 11 sub-domains characteristic of a typical protein kinase. (B) Expression and purification of LecRK-VI.2-KD from E.coli using affinity resin and phosphorylation assay. The GST-fusion protein was stained with Coomassie blue and confirmed by peptide sequencing. GST was used as a control (left panel). GST fusion protein LecRKVI.2-KD (2 µg) was incubated with ATP for 30 min in the presence of 5 mM MnCl2, 5 mM CaCl2 or 5 mM MgCl2. Phosphorylation signal was observed with ProQ Diamond phosphoprotein gel staining (right panel). (C) B. cinerea disease symptoms. Arabidopsis leaves were droplet-inoculated and symptoms were visualized 2 d later. Experiments were repeated 3 times with similar results. Bar = 1 cm. Error bars are SD (n = 18 leaves). Different letters indicate statistically significant differences compared with the wild-type Col-0 (LSD test; p < 0.05). (D) Relative expression levels of WRKY53, FRK1, NHL10, CYP81F2 and CBP60 g were analyzed 30 and 60 min after chitin infiltration (50 µg/ml). EF-1 and UBQ10 were used for normalization. Relative gene expression levels were compared with buffer control (defined value of 1) by qRT-PCR analyses. The values are the means ± SD of three biological replicates (n = 9). No significant differences to wild-type Col-0 were observed when based on a t-test (p < 0.01).
Conclusions
We showed that LecRKVI.2 is a positive regulator of bacterial PTI response in Arabidopsis.Citation8 In this report we demonstrate that LecRKVI.2 possesses a functional kinase domain. PTI, SA- and JA-mediated signaling cascades are intimately boundCitation1 and manipulation of PTI through alteration of LecRK-VI.2 expression may alter JA signaling and resistance to necrotrophic fungi. We therefore asked whether LecRKVI.2 plays a role in resistance to a necrotrophic fungal pathogen such as B. cinerea. Resistance to B. cinerea and upregulation of PTI-responsive genes after treatment with the fungal PAMP chitin were at wild-type levels in lecrk-VI.2-1. Similarly, the PTI response triggered by chitin does not depend on BAK1.Citation12 Collectively these observations indicate that LecRK-VI.2 is not required for basal resistance to B. cinerea and suggest that LecRK-VI.2 is specifically involved in bacterial defense signaling. LecRK-VI.2 may thus function as a positive regulator specific for bacteria-triggered PTI.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to M.R. Grant and the Arabidopsis Biological Resource Center for providing seeds. We thank C.Y. Chen for B. cinerea. We thank the staff of Technology Commons, College of Life Science at the National Taiwan University, for qRT-PCR equipment. This work was supported by the National Science Council of Taiwan grants 96-2628-B-002-112-MY3 and 99-2628-B-002-053-MY3 (to L.Z.).
References
- Tsuda K, Katagiri F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol 2010; 13:459 - 65; http://dx.doi.org/10.1016/j.pbi.2010.04.006; PMID: 20471306
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, et al. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci U S A 1998; 95:15107 - 11; http://dx.doi.org/10.1073/pnas.95.25.15107; PMID: 9844023
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 2003; 35:193 - 205; http://dx.doi.org/10.1046/j.1365-313X.2003.01794.x; PMID: 12848825
- Zimmerli L, Métraux JP, Mauch-Mani B. beta-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol 2001; 126:517 - 23; http://dx.doi.org/10.1104/pp.126.2.517; PMID: 11402183
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 2008; 20:471 - 81; http://dx.doi.org/10.1105/tpc.107.056754; PMID: 18263776
- De Smet I, Voss U, Jürgens G, Beeckman T. Receptor-like kinases shape the plant. Nat Cell Biol 2009; 11:1166 - 73; http://dx.doi.org/10.1038/ncb1009-1166; PMID: 19794500
- Bouwmeester K, Govers F. Arabidopsis L-type lectin receptor kinases: phylogeny, classification, and expression profiles. J Exp Bot 2009; 60:4383 - 96; http://dx.doi.org/10.1093/jxb/erp277; PMID: 19773388
- Singh P, Kuo YC, Mishra S, Tsai CH, Chien CC, Chen CW, et al. The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 2012; 24:1256 - 70; http://dx.doi.org/10.1105/tpc.112.095778; PMID: 22427336
- Joshi A, Dang HQ, Vaid N, Tuteja N. Pea lectin receptor-like kinase promotes high salinity stress tolerance in bacteria and expresses in response to stress in planta. Glycoconj J 2010; 27:133 - 50; http://dx.doi.org/10.1007/s10719-009-9265-6; PMID: 19898933
- Ouyang SQ, Liu YF, Liu P, Lei G, He SJ, Ma B, et al. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J 2010; 62:316 - 29; http://dx.doi.org/10.1111/j.1365-313X.2010.04146.x; PMID: 20128882
- Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 1995; 9:576 - 96; PMID: 7768349
- Shan L, He P, Li J, Heese A, Peck SC, Nürnberger T, et al. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 2008; 4:17 - 27; http://dx.doi.org/10.1016/j.chom.2008.05.017; PMID: 18621007