Abstract
In his recent opus magnum review paper published in the October issue of Physiology Reviews, Rainer Hedrich summarized the field of plant ion channels.1 He started from the earliest electric recordings initiated by Charles Darwin of carnivorous Dionaea muscipula,1,2 known as Venus flytrap, and covered the topic extensively up to the most recent discoveries on Shaker-type potassium channels, anion channels of SLAC/SLAH families, and ligand-activated channels of glutamate receptor-like type (GLR) and cyclic nucleotide-gated channels (CNGC).1
Keywords: :
It is interesting to recall that the earliest electric recordings on animal (squid neurons) cells were studied side-by side with plant (algae Nitella and Chara) cells. Pioneers of bioelectricity discovered that the plasma membrane of all eukaryotic cells is excitable and electrically active, despite some differences in ions and ion channels involved.Citation1 Some cells, especially neurons, are electrically more active and communicate via action potentials. Surprisingly, although very similar action potentials were recorded in plants already in 1873, the role of plant action potentials is still not settled in today. Nevertheless, it is obvious that not only the so-called sensitive plants, but in fact all plants generate action potentials (APs) and that these APs serve for communication and integration of plant bodies,Citation3-Citation5 which can attain extraordinary sizes in some trees. Asymmetric distributions of ions at cell periphery generates resting electric potential at the plasma membrane.Citation6 This is about -100 mV in typical animal cellsCitation6-Citation8 but higher in most plant cells, sometimes exceeding even -200 mV.Citation9 Interestingly, this resting electric potential at the plasma membrane is changing also along the root apex.Citation10 The next more complex feature of plant cells, in comparison to animal cells, is the presence of the vacuolar membrane which is also equipped with ion channels and generates its own electrochemical gradient.Citation1,Citation11 This results in trans-cytoplasmic potential of about -100 mV.Citation1
With respect to Venus flytrap, we know now that a single mechanical stimulus of sensory hairs induces an action potential (AP),Citation12 but the trap requires two repetitive stimuli (APs) to close and three repetitive APs are required to activate digestive glands.Citation13 Moreover, Venus flytrap uses electrical memory to control the behavior of its trap.Citation14,Citation15 Dionaea muscipula traps and Mimosa pudica leaves get immobilized when exposed to anesthetics.Citation16,Citation17 Importantly, they can be easily recovered to behavioral activity by removing anesthetics from their environment. All this suggest fascinating similarities between plants and animals with respect of APs driving motoric behavior. Intriguingly, numerous examples of sophisticated plant behavior implicate existence of plant-specific consciousness and intelligence too.Citation19
Surprisingly, recent genomic studies have revealed unexpected ligand-activated channels of GLR and CNGCs families; and, moreover, these animal-like channels outnumbered the well-studied potassium channels. For example, there are 15 potassium channels in Arabidopsis, but as many as 20 GLRs and 20 CNGCs.Citation18 Even greater differences have been scored in larger and more complex plants like poplar tree: 15 potassium channels vs. 61 GLRs.Citation20 Perhaps the greatest mystery is associated with the plant GLRs. Although they were discovered more than ten years ago, we still know very little about their localization and function. If these are used in analogy to the animal/neuronal GLRs then one could expect that plant cells assemble synaptic domains specialized for cell-cell communication, analogously to neurons in brains. Accordingly, root apex cells in the transition zone not only shows F-actin-based adhesion domains specialized for endocytosis, endocytic vesicle recycling, and cell-cell communication; but they also show high rates of APs and synchronous electrical firing.Citation21-Citation23 In neurons organized into brains, cell-cell communication is based on endocytic recycling and regulated exocytosis of synaptic vesicles, when synaptotagmin acts as calcium sensor.Citation24 Intriguingly in this respect, plant-specific synaptotagmin AtSYT1 localizes to the plant-specific synaptic domains of Arabidopsis root apices too,Citation25 and controls besides exocytosis also endocytosis.Citation26 Our preliminary data suggest that Arabidopsis GLRs control endocytic vesicle recycling in the transition zoner cells of root apices of young Arabidopsis seedlings (Matthias Weiland, Boris Voigt and František Baluška, unpublished data).
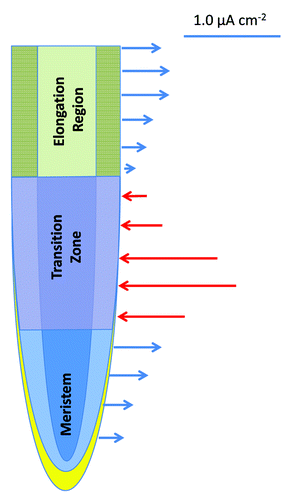
Activities of ion channels generate electric fields around the plasma membrane, whereas a sum of all ion channels activities in a given zone of the root apex is responsible for patterns of electric fields around the whole root apex,Citation27,Citation28 like in animals and humans. For example, in human brains, summations of synchronous ion channel activities of all neurons generate characteristic electric fields which can be measured using electroencephalography (EEG).Citation29,Citation30 Although these extracellular electric fields around plant roots are generated by H+ and K+ fluxes, activities of ligand-activated ion channels are also involved in the generation of brain EEG.Citation29 In the growing root apices of all plants tested, there are three stable distinct zones of electric current flow (): outward currents are recorded around the root cap, meristem and the elongation region, whereas prominent inward currents are scored at the transition zone.Citation27,Citation28 Thus, there are two sudden reversals of the electric current polarity around growing root apex: one is the outward - inward switch when cells cease mitotic divisions and enter the transition zone; another one is the inward - outward switch when cells leave the transition zone and enter the region of rapid cell elongation.Citation28 Currently, there is no explanation for these sudden reversals of the electric current polarity in the transition zoneCitation31-Citation35 of the root apex. Transport activities are not only synchronized in the transition zone, but show also oscillations.Citation37-Citation40 In addition, root apex electric fieldsCitation41,Citation42 and gene expression activitiesCitation43,Citation44 are also known to oscillate in this root apex zone. In future, it will be important to test whether activities of the GLRs and CNGCs are behind these imtriguing aspects of the root apex transition zone. The fact that all GLRs, and most of the CNGCs, are expressed in roots of ArabidopsisCitation18,Citation36 is particularly relevant in this respect.
Figure 1. Schematic visualization of the electric field around the growing maize root apex using data published by Collings et al.Citation28 The outward current is shown by blue arrows, the inward current is depicted by red arrows. The two reversals of the electric current polarity at the both borders of the transition zone are typical for root apices of all plants tested so far. Identity of ion channels underlying this phenomenon is enigmatic so far, but they do not seem to be related to fluxes of H+ and K+. The GLRs and CNGCs are strong candidates in this respect.
Recently, the first breakthrough studies have started to illuminate the elusive functions of GLRs in plants. First of all, Michard et al.Citation45 reported that the plant GLRs in pollen tubes of Arabidopsis and tobacco act similarly as the neuronal glutamate receptors. They are activated by their ligands to transport calcium into cells in a signaling-like mode, allowing a signal-mediated calcium influx, which then controls pollen tube tip-growth and navigation.Citation45 Interestingly, GLRs in the tip-growing pollen tube are activated via d-serine, which is released from pistil tissues. D-Serine is produced from L-Serine via Arabidopsis serine-racemase SR1.Citation45 This milestone paper suggests that pollination in plants is a neuronal-like process in which pollen tubes are navigated through pistil tissues via neuronal-like cell-cell communication. This view is supported also by previous papers showing that navigation of pollen tubes is also controlled by NO and GABA.Citation46-Citation49 Hence, it is particularly relevant that GABA-binding proteins have been detected at the pollen plasma membrane which are relevant for calcium transport and oscillations.Citation50
Plant GLRs are now recognized to have also important role in plant – pathogen interactions. It is well known that diverse pathogens induce calcium fluxes and generate calcium spikes in plant cells exposed to pathogens or relevant microbe-associated molecular pattern (MAMPs) such as flagellar proteins, chitin fragments, elongation factors, or secreted peptide elicitors. These MAMPs are then recognized by cell surface receptors, which signal downstream via calcium influx to alert the attacked cells. For example, flagellin is recognized by the receptor kinase FLS2, which interact with BAK1 (BRI1-associated receptor kinase 1)Citation51 to activate calcium influx, rapid plasma membrane depolarization and H2O2 burst.Citation1 All this happens within the first two minutes of flagellin exposure.Citation1 Intriguingly, plant GLRs are participating in this MAMP-triggered calcium influx,Citation52,Citation53 and analogous to mammalian NMDA-NR1 receptor, calmodulin is also involved.Citation53 The next breakthrough is the discovery that plant GLRs are activated by elicitor cryptogein, being partly responsible for cryptogein-induced NO production.Citation54
Intriguingly, cryptogein-induced brefeledin A-sensitive vesicular secretion of glutamate into the apoplastCitation54 suggests that similarly secreted glutamate might activate GLRs also in plant tissues.
In future, it will be important to study possible roles of GLRs and CNGCs, as well as of secreted L-glutamate and d-serine, in the context of secretory-active polar plant cells, organized into longitudinal cell files of plant organs such as root apex; generating its own bioelecteric fields.
References
- Hedrich R. Ion channels in plants. Physiol Rev 2012; 92:1777 - 811; http://dx.doi.org/10.1152/physrev.00038.2011; PMID: 23073631
- Burdon-Sanderson J. Venus fly-trap (Dionaea muscipula). Nature 1874; 10:105 - 7, 127-8; http://dx.doi.org/10.1038/010105c0
- Davies E. Action potentials as multifunctional signals in plants: a unifying hypothesis to explain apparently disparate wound. Plant Cell Environ 1987; 10:623 - 31; http://dx.doi.org/10.1111/j.1365-3040.1987.tb01844.x
- Felle HH, Zimmermann MR. Systemic signalling in barley through action potentials. Planta 2007; 226:203 - 14; http://dx.doi.org/10.1007/s00425-006-0458-y; PMID: 17226028
- Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environ 2007; 30:249 - 57; http://dx.doi.org/10.1111/j.1365-3040.2006.01614.x; PMID: 17263772
- Veech RL, Kashiwaya Y, Gates DN, King MT, Clarke K. The energetics of ion distribution: the origin of the resting electric potential of cells. IUBMB Life 2002; 54:241 - 52; http://dx.doi.org/10.1080/15216540215678; PMID: 12587974
- Bezanilla F. The action potential: from voltage-gated conductances to molecular structures. Biol Res 2006; 39:425 - 35; http://dx.doi.org/10.4067/S0716-97602006000300005; PMID: 17106575
- Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol 2008; 9:323 - 32; http://dx.doi.org/10.1038/nrm2376; PMID: 18354422
- Pedersen CNS, Axelsen KB, Harper JF, Palmgren MG. Evolution of plant p-type ATPases. Front Plant Sci 2012; 3:31; http://dx.doi.org/10.3389/fpls.2012.00031; PMID: 22629273
- Illés P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovecka M. Aluminium toxicity in plants: internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot 2006; 57:4201 - 13; http://dx.doi.org/10.1093/jxb/erl197; PMID: 17085753
- Bethmann B, Thaler M, Simonis W, Schonknecht G. Electrochemical potential gradients of H+, K+, Ca2+, and Cl- across the tonoplast of the green alga Eremosphaera viridis.. Plant Physiol 1995; 109:1317 - 26; PMID: 12228672
- Volkov AG, Adesina T, Jovanov E. Closing of venus flytrap by electrical stimulation of motor cells. Plant Signal Behav 2007; 2:139 - 45; http://dx.doi.org/10.4161/psb.2.3.4217; PMID: 19516982
- Escalante-Pérez M, Krol E, Stange A, Geiger D, Al-Rasheid KA, Hause B, et al. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proc Natl Acad Sci U S A 2011; 108:15492 - 7; http://dx.doi.org/10.1073/pnas.1112535108; PMID: 21896747
- Volkov AG, Carrell H, Baldwin A, Markin VS. Electrical memory in Venus flytrap. Bioelectrochemistry 2009; 75:142 - 7; http://dx.doi.org/10.1016/j.bioelechem.2009.03.005; PMID: 19356999
- Volkov AG, Carrell H, Adesina T, Markin VS, Jovanov E. Plant electrical memory. Plant Signal Behav 2008; 3:490 - 2; http://dx.doi.org/10.4161/psb.3.7.5684; PMID: 19704496
- Milne A, Beamish T. Inhalational and local anesthetics reduce tactile and thermal responses in mimosa pudica.. Can J Anaesth 1999; 46:287 - 9; http://dx.doi.org/10.1007/BF03012612; PMID: 10210057
- De Luccia TP. Mimosa pudica, Dionaea muscipula and anesthetics. Plant Signal Behav 2012; 7:1163 - 7; http://dx.doi.org/10.4161/psb.21000; PMID: 22899087
- Dietrich P, Anschütz U, Kugler A, Becker D. Physiology and biophysics of plant ligand-gated ion channels. Plant Biol (Stuttg) 2010; 12:Suppl 1 80 - 93; http://dx.doi.org/10.1111/j.1438-8677.2010.00362.x; PMID: 20712623
- Trewavas AJ, Baluška F. The ubiquity of consciousness. EMBO Rep 2011; 12:1221 - 5; http://dx.doi.org/10.1038/embor.2011.218; PMID: 22094270
- Ward JM, Mäser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 2009; 71:59 - 82; http://dx.doi.org/10.1146/annurev.physiol.010908.163204; PMID: 18842100
- Baluska F, Volkmann D, Menzel D. Plant synapses: actin-based domains for cell-to-cell communication. Trends Plant Sci 2005; 10:106 - 11; http://dx.doi.org/10.1016/j.tplants.2005.01.002; PMID: 15749467
- Baluska F, Mancuso S, Volkmann D, Barlow PW. Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 2010; 15:402 - 8; http://dx.doi.org/10.1016/j.tplants.2010.04.007; PMID: 20621671
- Masi E, Ciszak M, Stefano G, Renna L, Azzarello E, Pandolfi C, et al. Spatiotemporal dynamics of the electrical network activity in the root apex. Proc Natl Acad Sci U S A 2009; 106:4048 - 53; http://dx.doi.org/10.1073/pnas.0804640106; PMID: 19234119
- Koch M, Holt M. Coupling exo- and endocytosis: An essential role for PIP2 at the synapse. Biochim Biophys Acta 2012; 1821:1114-32.
- Schapire AL, Voigt B, Jasik J, Rosado A, Lopez-Cobollo R, Menzel D, et al. Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. Plant Cell 2008; 20:3374 - 88; http://dx.doi.org/10.1105/tpc.108.063859; PMID: 19088329
- Lewis JD, Lazarowitz SG. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc Natl Acad Sci U S A 2010; 107:2491 - 6; http://dx.doi.org/10.1073/pnas.0909080107; PMID: 20133785
- Iwabuchi A, Yano M, Shimizu H. Development of extracellular electric pattern around Lepidium roots: its possible role in root growth and gravitropism. Protoplasma 1989; 148:94 - 100; http://dx.doi.org/10.1007/BF02079327
- Collings DA, White RG, Overall RL. Ionic current changes associated with the gravity-induced bending response in roots of Zea mays L. Plant Physiol 1992; 100:1417 - 26; http://dx.doi.org/10.1104/pp.100.3.1417; PMID: 16653140
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci 2012; 13:407 - 20; http://dx.doi.org/10.1038/nrn3241; PMID: 22595786
- Whittingstall K, Logothetis NK. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron 2009; 64:281 - 9; http://dx.doi.org/10.1016/j.neuron.2009.08.016; PMID: 19874794
- Baluška F, Volkmann D, Barlow PW. Specialized zones of development in roots: view from the cellular level. Plant Physiol 1996; 112:3 - 4; PMID: 11536754
- Baluška F, Volkmann D, Barlow PW. A polarity crossroad in the transition growth zone of maize root apices: cytoskeletal and developmental implications. J Plant Growth Regul 2001; 20:170 - 81; http://dx.doi.org/10.1007/s003440010013
- Verbelen J-P, De Cnodder T, Le J, Vissenberg K, Baluška F. The root apex of Arabidopsis thaliana consists of four distinct zones of cellular activities: meristematic zone, transition zone, fast elongation zone, and growth terminating zone. Plant Signal Behav 2006; 1:296 - 304; http://dx.doi.org/10.4161/psb.1.6.3511; PMID: 19517000
- Baluška F, Mancuso S, Volkmann D, Barlow PW. Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 2010; 15:402 - 8; http://dx.doi.org/10.1016/j.tplants.2010.04.007; PMID: 20621671
- Ivanov VB, Dubrovsky JG. Longitudinal zonation pattern in plant roots: conflicts and solutions. Trends Plant Sci 2012; In press http://dx.doi.org/10.1016/j.tplants.2012.10.002; PMID: 23123304
- Roy SJ, Gilliham M, Berger B, Essah PA, Cheffings C, Miller AJ, et al. Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana.. Plant Cell Environ 2008; 31:861 - 71; http://dx.doi.org/10.1111/j.1365-3040.2008.01801.x; PMID: 18284583
- Mancuso S, Boselli M. Characterisation of the oxygen fluxes in the division, elongation and mature zones of Vitis roots: influence of oxygen availability. Planta 2002; 214:767 - 74; http://dx.doi.org/10.1007/s004250100670; PMID: 11882946
- Mancuso S, Marras AM, Magnus V, Baluška F. Noninvasive and continuous recordings of auxin fluxes in intact root apex with a carbon nanotube-modified and self-referencing microelectrode. Anal Biochem 2005; 341:344 - 51; http://dx.doi.org/10.1016/j.ab.2005.03.054; PMID: 15907881
- McLamore ES, Diggs A, Calvo Marzal P, Shi J, Blakeslee JJ, Peer WA, et al. Non-invasive quantification of endogenous root auxin transport using an integrated flux microsensor technique. Plant J 2010; 63:1004 - 16; http://dx.doi.org/10.1111/j.1365-313X.2010.04300.x; PMID: 20626658
- Mugnai S, Azzarello E, Baluška F, Mancuso S. Local root apex hypoxia at the transition zone induces NO-mediated hypoxic acclimation of the whole root. Plant Cell Physiol 2012; 53:912 - 20; http://dx.doi.org/10.1093/pcp/pcs034; PMID: 22422934
- Scott BIH. Electric oscillations generated by plant roots and a possible feedback mechanism responsible for them. Aust J Biol Sci 1957; 10:164 - 79
- Jenkinson IS, Scott BIH. Bioelectric oscillations of bean roots: further evidence for a feedback oscillator. I. Extracellular response to oscillations in osmotic pressure and auxin. Aust J Biol Sci 1961; 14:231 - 47
- Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 2010; 329:1306 - 11; http://dx.doi.org/10.1126/science.1191937; PMID: 20829477
- Moreno-Risueno MA, Benfey PN. Time-based patterning in development: The role of oscillating gene expression. Transcription 2011; 2:124 - 9; http://dx.doi.org/10.4161/trns.2.3.15637; PMID: 21826283
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, et al. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011; 332:434 - 7; http://dx.doi.org/10.1126/science.1201101; PMID: 21415319
- Prado AM, Porterfield DM, Feijó JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 2004; 131:2707 - 14; http://dx.doi.org/10.1242/dev.01153; PMID: 15128654
- Prado AM, Colaço R, Moreno N, Silva AC, Feijó JA. Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signaling. Mol Plant 2008; 1:703 - 14; http://dx.doi.org/10.1093/mp/ssn034; PMID: 19825574
- Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003; 114:47 - 59; http://dx.doi.org/10.1016/S0092-8674(03)00479-3; PMID: 12859897
- Yu GH, Sun MX. Deciphering the possible mechanism of GABA in tobacco pollen tube growth and guidance. Plant Signal Behav 2007; 2:393 - 5; http://dx.doi.org/10.4161/psb.2.5.4265; PMID: 19704611
- Yu G, Liang J, He Z, Sun M. Quantum dot-mediated detection of gamma-aminobutyric acid binding sites on the surface of living pollen protoplasts in tobacco. Chem Biol 2006; 13:723 - 31; http://dx.doi.org/10.1016/j.chembiol.2006.05.007; PMID: 16873020
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007; 448:497 - 500; http://dx.doi.org/10.1038/nature05999; PMID: 17625569
- Kwaaitaal M, Huisman R, Maintz J, Reinstädler A, Panstruga R. Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana.. Biochem J 2011; 440:355 - 65; http://dx.doi.org/10.1042/BJ20111112; PMID: 21848515
- Kwaaitaal M, Maintz J, Cavdar M, Panstruga R. On the ligand binding profile and desensitization of plant ionotropic glutamate receptor (iGluR)-like channels functioning in MAMP-triggered Ca2+ influx. Plant Signal Behav 2012; 7:1373 - 7; http://dx.doi.org/10.4161/psb.21761
- Vatsa P, Chiltz A, Bourque S, Wendehenne D, Garcia-Brugger A, Pugin A. Involvement of putative glutamate receptors in plant defence signaling and NO production. Biochimie 2011; 93:2095 - 101; http://dx.doi.org/10.1016/j.biochi.2011.04.006; PMID: 21524679