Abstract
Auxins and brassinosteroids are essential phytohormones that synergistically regulate physiological and developmental processes in plants. Previously, we demonstrated that auxins stimulate brassinosteroid perception by regulating the level of brassinosteroid receptor in rice. Here we showed that auxin treatment increased expression of the Arabidopsis brassinosteroid receptor gene BRI1. The promoter of BRI1 has an auxin-response element that is targeted by auxin-response factor transcription factors. Auxin pretreatment increased the sensitivity to brassinosteroids of brassinosteroid-responsive genes. Although multilevel interactions between auxins and brassinosteroids have previously been reported, our findings suggest a possibility that auxins control the degree of brassinosteroid perception by regulating the expression of gene for brassinosteroid receptor, and this phenomenon is conserved between monocots (rice) and dicots (Arabidopsis).
In plants, phytohormones govern every aspect of growth and development. Many physiological phenomena are regulated either synergistically or antagonistically by multiple phytohormones, including auxins and brassinosteroids. Auxins and brassinosteroids have synergistic effects in the promotion of leaf lamina inclination (the degree of upright leaf angle) in rice, and in a previous report,Citation1 we showed that auxins stimulate brassinosteroid perception by regulating the level of brassinosteroid receptor in rice. Auxin treatment increased expression of the rice brassinosteroid receptor gene OsBRI1, of which promoter has an auxin-response element (AuxRE; TGTCTC)Citation2 that is targeted by auxin-response factor (ARF) transcription factors. Because the AuxRE in the OsBRI1 promoter and the upregulation of OsBRI1 expression caused by auxin treatment are essential for an auxin-induced increase in sensitivity to brassinosteroids, we concluded that some activator ARFs control the degree of brassinosteroid perception required for normal growth and development in rice.
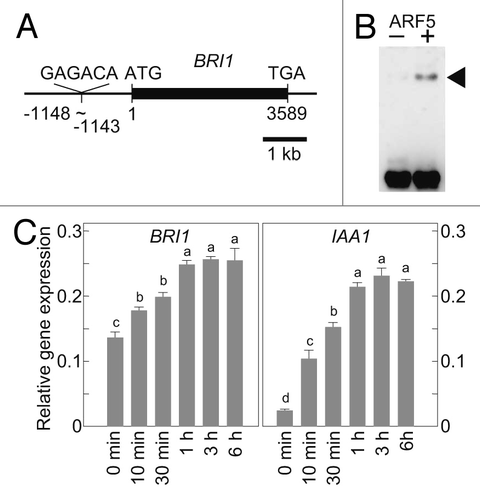
An auxin-induced increase in brassinosteroid receptor gene expression was also observed in Arabidopsis.Citation3 We identified an AuxRE motif in the 5′-flanking region of the Arabidopsis brassinosteroid receptor gene BRI1 (the inverted sequence of TGTCTC, GAGACA; ). An electrophoresis mobility shift assay revealed that the recombinant Arabidopsis activator ARF protein ARF5Citation4 expressed in Escherichia coli cells could bind to a 66 bp BRI1 promoter fragment containing AuxRE (). We also showed that indole-3-acetic acid (IAA, a bioactive auxin) treatment increased the BRI1 mRNA level. A significant increase in the level of BRI1 mRNA occurred within 10 min after 20 μM IAA treatment, and after 1 h, it had doubled to that in the untreated plants (). In contrast to OsBRI1 in rice, whose expression doubled within 1 h, and then returned to untreated levels by 3 h after 20 μM IAA treatment,Citation1 the increased level of BRI1 mRNA in Arabidopsis was maintained for at least 6 h after IAA treatment. An increased mRNA level was also maintained for an early auxin-responsive gene IAA1Citation5 in Arabidopsis treated with IAA (). Differences in the kinetics of the increase in gene expression between BRI1 and OsBRI1 may reflect the differences in auxin response between rice and Arabidopsis. Anyway, these results suggest a possibility that BRI1 expression is regulated by auxins and activator ARF proteins in Arabidopsis, as was the case in rice.
Figure 1. Auxins increase the expression of BRI1 in Arabidopsis. (A) Genomic structure of ArabidopsisBRI1. An inverted AuxRE sequence (GAGACA) was found in the promoter region of BRI1 at positions -1148 to -1143 (taking the translation initiation site as +1). (B) Recombinant Arabidopsis ARF5 protein binds to a BRI1 promoter fragment containing AuxRE. Arrowhead indicates the ARF5-DNA complex. (C) Quantitative RT-PCR analysis of BRI1 and IAA1 expression in one-week-old Arabidopsis seedlings treated with 20 μM IAA. Numbers under the bars indicate the time after IAA treatment. Expression levels were normalized against the values obtained for TUBULIN. Values are the means ± SD of three biological repeats. Bars labeled with different letters differ significantly (Tukey HSD test, p < 0.05).
Next, we examined the effect of auxins on the mRNA levels of two Arabidopsis genes, At1g62440 and At3g50560, both of which are induced by 1 μM brassinolide (BL, a bioactive brassinosteroid) treatment for 2.5 h but not by 10 μM IAA treatment for 2.5 h.Citation3 If the IAA-induced increase in BRI1 expression enhanced the brassinosteroid sensitivity, we would expect that IAA treatment would at least transiently increase At1g62440 and At3g50560 expressions without the exogenous treatment of brassinosteroids. As expected, 20 μM IAA treatment transiently increased At1g62440 and At3g50560 expressions to 1.8 and 2.2 times, respectively, that in untreated plants (). However, these two gene expressions did not increase significantly until about 1 h after IAA treatment. By comparison, the increase in levels of BRI1 mRNA began within 10 min after IAA treatment (). The delay in the increase in At1g62440 and At3g50560 expressions suggests that IAA treatment indirectly induces these brassinosteroid-responsive gene expressions.
Figure 2. Auxins increase the expression of Arabidopsis brassinosteroid-responsive genes. (A) Quantitative RT-PCR analysis of two brassinosteroid-responsive genes, At1g62440 and At3g50560, in one-week-old Arabidopsis seedlings treated with 20 μM IAA. Numbers under the bars indicate the time after IAA treatment. (B) Quantitative RT-PCR analysis of At1g62440 and At3g50560 expression in BL-treated one-week-old Arabidopsis seedlings pretreated with 20 μM IAA 4 h before the 1 μM BL treatment. Seedlings were washed just before BL treatment to remove the IAA. Numbers under the bars indicate the number of hours after BL treatment. Expression levels were normalized against the values obtained for TUBULIN. Values are the means ± SD of three biological repeats. Bars labeled with different letters differ significantly (Tukey HSD test, p < 0.05).
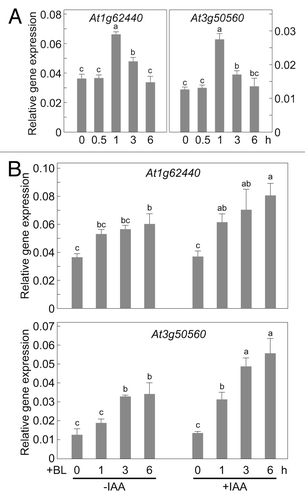
We also examined the effect of auxin pretreatment on brassinosteroid-induced At1g62440 and At3g50560 expressions. Without IAA pretreatment, At1g62440 and At3g50560 expressions were gradually increased by BL treatment to 1.6 and 2.7 times, respectively, that in untreated plants after 6 h (, -IAA), whereas At1g62440 and At3g50560 expressions in IAA-pretreated plants were 2.2 and 4.1 times, respectively, of the level in untreated plants by 6 h after BL treatment (, +IAA). These results demonstrated that IAA pretreatment significantly increases sensitivity to brassinosteroids and downstream brassinosteroid-responsive gene expressions.
It has been demonstrated that BIN2 kinase negatively regulates brassinosteroid signaling by phosphorylating the BES1 and BZR1 transcription factors, and changes in the phosphorylation status of BES1 and BZR1 alter their activity and thereby result in altered expression levels of their target brassinosteroid-responsive genes.Citation6-Citation10 However, the accumulation of unphosphorylated BES1 and BZR1 was not observed after auxin treatment.Citation6,Citation7 These findings suggest a possibility that auxins can induce BRI1 expression in Arabidopsis but do not stimulate the brassinosteroid signaling. However, we hypothesized that the IAA-induced increase in BRI1 mRNA, which was about twice that in untreated seedlings, would affect brassinosteroid signaling. Indeed, IAA pretreatment significantly increased the level of At1g62440 and At3g50560 mRNAs after BL treatment. We therefore believe that the auxin-induced increase in brassinosteroid sensitivity is an early event in transcriptional auxin-brassinosteroid synergism, and this striking phenomenon is conserved between monocots (rice) and dicots (Arabidopsis). A recent study in Arabidopsis showed that auxins enhanced brassinosteroid biosynthesis, in part by inhibiting BZR1 feedback repression of the DWARF4 gene, which encodes a C-22 hydroxylase that is involved in a rate-limiting step in brassinosteroid biosynthesis.Citation11 Further analysis is needed to understand the mechanism by which auxins increases the brassinosteroid response in Arabidopsis, our present results support the notion that interactions between auxins and brassinosteroids are extensive and complex.
| Abbreviations: | ||
| ARF | = | auxin-response factor |
| AuxRE | = | auxin-response element |
| BL | = | brassinolide |
| IAA | = | indole-3-acetic acid |
Acknowledgments
T.S. was supported by Grants-in-Aid for Young Scientists (nos. 19688001 and 24780005) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. S.F. was supported by Grants-in-Aid for Scientific Research (B) (nos. 19380069 and 23380066) from MEXT.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Sakamoto T, Morinaka Y, Inukai Y, Kitano H, Fujioka S. Auxin signal transcription factor regulates expression of the brassinosteroid receptor gene in rice. Plant J 2012; Epub ahead of print http://dx.doi.org/10.1111/tpj.12071; PMID: 23146214
- Ulmasov T, Liu Z-B, Hagen G, Guilfoyle TJ. Composite structure of auxin response elements. Plant Cell 1995; 7:1611 - 23; PMID: 7580254
- Nemhauser JL, Mockler TC, Chory J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis.. PLoS Biol 2004; 2:E258; http://dx.doi.org/10.1371/journal.pbio.0020258; PMID: 15328536
- Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev 2002; 16:1610 - 5; http://dx.doi.org/10.1101/gad.229402; PMID: 12101120
- Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana.. J Mol Biol 1995; 251:533 - 49; http://dx.doi.org/10.1006/jmbi.1995.0454; PMID: 7658471
- He JX, Gendron JM, Yang Y, Li J, Wang ZY. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis.. Proc Natl Acad Sci U S A 2002; 99:10185 - 90; http://dx.doi.org/10.1073/pnas.152342599; PMID: 12114546
- Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, et al. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002; 109:181 - 91; http://dx.doi.org/10.1016/S0092-8674(02)00721-3; PMID: 12007405
- Gendron JM, Wang ZY. Multiple mechanisms modulate brassinosteroid signaling. Curr Opin Plant Biol 2007; 10:436 - 41; http://dx.doi.org/10.1016/j.pbi.2007.08.015; PMID: 17904409
- Li J, Jin H. Regulation of brassinosteroid signaling. Trends Plant Sci 2007; 12:37 - 41; http://dx.doi.org/10.1016/j.tplants.2006.11.002; PMID: 17142084
- Kim TW, Wang ZY. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 2010; 61:681 - 704; http://dx.doi.org/10.1146/annurev.arplant.043008.092057; PMID: 20192752
- Chung Y, Maharjan PM, Lee O, Fujioka S, Jang S, Kim B, et al. Auxin stimulates DWARF4 expression and brassinosteroid biosynthesis in Arabidopsis. Plant J 2011; 66:564 - 78; http://dx.doi.org/10.1111/j.1365-313X.2011.04513.x; PMID: 21284753