Abstract
For over a century, plant fertilization has been thought to depend on the fertility of a single pollen tube. However, we reported recently a “fertilization recovery system” in flowering plants that actively rescues failed fertilization of a defective mutant pollen tube by attracting a second, functional pollen tube. In typical flowering plants, two synergid cells beside the egg cell attract pollen tubes, one of which degenerates upon pollen tube discharge. We observed that fertilization was rescued when the second synergid cell accepted a wild-type pollen tube. Our results suggest that flowering plants precisely control the number of pollen tubes that arrive at each ovule and use a fertilization recovery mechanism to maximize the likelihood of successful seed set. Restricted pollination experiments showed that if sufficient pollen grains are provided, ovules attract a second pollen tube for recovery. These results support our previous finding that a long period of time is required for ovules to complete the system.
In angiosperms, double fertilization takes place within the ovule with the entry of two sperm cells, usually delivered by a single pollen tube. In 1904, Wylie observed that two pollen tubes were inserted in an ovule in Elodea canadensis.Citation1 He concluded, “It often happens that two pollen tubes pass into one ovule; in such cases both synergids disappear.” After Wylie’s discovery, rare cases of the reception of two pollen tubes in an embryo sac have been reported in at least 12 species.Citation2 Similarly, the reception of two pollen tubes has been reported in several Arabidopsis mutants, including the gcs1 mutant.Citation3
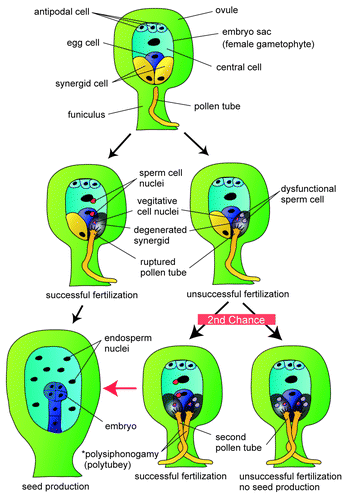
Although this phenomenon is interesting, it has long been regarded as an anomalous event. As shown in , when we observed ovules frequently accepting two pollen tubes in the hap2-1 (allelic to gcs1) mutant,Citation4 which is defective in fertilization, we investigated the mechanism underlying this phenomenon in higher plants. We double-stained +/hap2-1 pollen tubes for GUS activity followed by aniline blue staining to trace the behavior of the first and second pollen tubes. Since hap2-1 mutant pollen tubes are marked by the pollen tube-specific reporter gene, LAT52:GUS,Citation5 we could trace the behavior of hap2-1 mutant pollen tubes in vivo.Citation4,Citation6 We showed that most ovules have one pollen tube 10 h after pollination (HAP), indicating that until several hours after the arrival of the first pollen tube, reception of a second pollen tube is independent of the fertility of sperm cells. This delay could represent a blocking system by which ovules avoid polysiphonogamy ().Citation7 After 10 HAP, a second pollen tube starts to be attracted by ovules that failed to be fertilized with the first pollen tube of the hap2-1 allele. In this case, the persistent synergid cell, which degenerates upon successful fertilization, continues to attract pollen tubes,Citation8 resulting in ~80% of failed ovules accepting a second pollen tube by 28 HAP. No particular role had been proposed for the persistent synergid cell after the arrival of the first pollen tube. However, we demonstrated that the second synergid cell can retain its function and is able to attract and accept a second tube to rescue fertilization. This might explain the presence of two synergid cells in many higher plants ().
Figure 1. Two pollen tubes inserted in a single ovule. Scanning electron micrograph of wild-type ovules crossed with +/hap2-1 pollen. Two pollen tubes (colored red and blue) grow along the funiculus and then enter the micropyle.
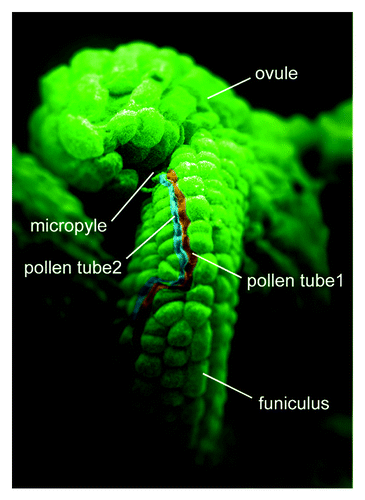
Figure 2. Fertilization recovery system and polysiphonogamy. Schematic drawing of the fertilization recovery system. Upon insertion of a single pollen tube into an ovule, the pollen tube bursts and releases two sperm cells. When the sperm cells complete fertilization, the ovule blocks the entry of the other pollen tubes and develops into a seed by forming an embryo and endosperm. When fertilization fails, the ovule attracts a second pollen tube to rescue fertilization. The rescued ovule develops into a seed, resulting in increased fertility. In the case of failure of fertilization by the second pollen tube, the ovule does not attract a third pollen tube, possibly due to depletion of the pollen tube attractant from synergid cells, since both synergid cells collapse after entry of two pollen tubes. *Polysiphonogamy and Polytubey: In angiosperms, a pollen tube delivers non-motile sperm cells accurately to the female gametophyte and completes fertilization. This is called “siphonogamy,” a mechanism that is thought to have evolved from zooidgamy (fertilization by motile sperm).Citation13 We use the term “polysiphonogamy” for cases in which an ovule accepts multiple pollen tubes and these tubes deliver sperm cells to the female gametophyte. In Arabidopsis, polysiphonogamy is restricted to two pollen tubes because only two synergid cells are present. In Amborella,Citation14 polysiphonogamy with three pollen tubes has been reported,Citation15 since three synergid cells are present. Polytubey is when an ovule accepts two or more pollen tubes, irrespective of whether fertilization occurs. In contrast, polysiphonogamy is one case of polytubey, in which fertilization by multiple pollen tubes is emphasized.
Previously,Citation7 we clarified why several mutants defective in sperm cells had an enhanced fertility phenotype (60–70% fertility).Citation4,Citation9,Citation10 The ratio of double pollen tube reception was almost completely consistent with the ratio of enhanced fertility in the male mutants. In addition, in the GUS staining experiment, 10 HAP, ~50% of the ovules accepted a mutant allele, suggesting that mutant and wild-type pollen tubes were similarly competent to enter the embryo sac and release their contents. von Besser et al.Citation4 suggested that hap2-1 sperm cells affect pollen tube guidance. From our data, however, we concluded that the sperm cells appear to be passive cargo of the pollen tube and do not influence pollen tube guidance in hap2 mutants. This was consistent with the observation that sperm-cell-defective mutants that cannot be transmitted via the male germline show only 30–35% sterility, instead of the expected 50%.Citation7
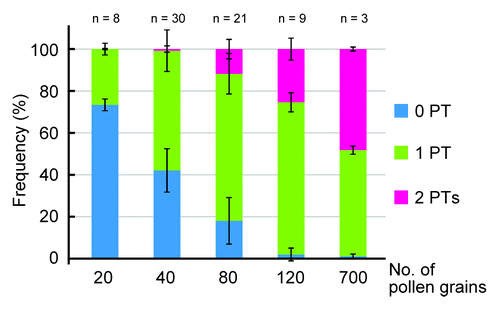
In our previous report, all hand pollination experiments were performed using large numbers of pollen grains, with a mean of 708 ± 111 pollen grains on the stigmata (mean ± S.D., n = 5 pistils). Under such conditions we were able to determine why an ovule accepts two pollen tubes, but it was impossible to answer how many pollen tubes are required for the recovery system to start accepting a second pollen tube. Therefore, we performed a restricted pollination experiment (). For the male parent, we pollinated the hap2-1 mutant pollen with the quartetCitation11 background to give a uniform number of pollen grains and equalize the wild-type to mutant pollen ratio; 1:1. An Arabidopsis pistil usually contains 50–60 ovules; we pollinated them with 20, 40, 80, 120 and ~700 grains. Two days after pollination, when 20 and 40 grains were provided (), few second pollen tubes was inserted into an ovule, indicating that under restricted conditions (ovules > pollen tubes), pollen tubes were selectively inserted into ovules that had not accepted any pollen tube. Conversely, when a wild-type pistil with 52.4 ± 6.1 ovules was pollinated with 80 grains (mean ± S.D.; n = 21 pistils, ovules < pollen tubes), 11.8 ± 4.6% of the ovules accepted second pollen tubes. When a wild-type pistil with 57.5 ± 4.9 ovules was pollinated with 120 grains (ovules < pollen tubes), 25.4 ± 5.2% of the ovules accepted second pollen tubes. These results suggest that pollen tubes are preferentially inserted into ovules with no pollen tube under restricted conditions (ovules > pollen tubes), while under saturated conditions (ovules < pollen tubes), ovules accept second pollen tubes. In our previous report,Citation7 when a wild-type pistil was pollinated with excess pollen grains (~700; ovules < pollen tubes), ~80% of the failed ovules accepted second pollen tubes. In addition, under self-pollination conditions, ~80% of the failed ovules accepted second pollen tubes. Under self-pollinated conditions, there were 191 ± 27 pollen grains on the stigmata. These results indicate that excess pollen is required for saturation of the fertilization recovery system and ~80%, but not 100%, of the failed ovules can accept a second pollen tube and complete recovery. These results support our previous finding that it takes ~28 h, a substantial time, for ovules to complete the fertilization recovery system. The reason for the delay in the second guidance may be the number of functional synergid cells. An ovule penetrated by a pollen tube contains only one persistent synergid cell, since one of the synergid cells is disrupted when the pollen tube bursts. In this case, the second guidance is governed by only one synergid cell. Higashiyama et al.Citation12 reported that an ovule with two synergid cells attracts more pollen tubes than one with one synergid cell, suggesting that the attractant is insufficient for ovules with one synergid cell. This could be one of the reasons why only ~80%, and not 100%, of the ovules with one synergid cell attract a second pollen tube. Further research into this system, for example, increasing the number of synergid cells experimentally or the expression of the pollen tube attractant, might provide new insight that can be applied to increase fertility and production in agronomically important plants.
Figure 3. Fertilization recovery system requires an excess of pollen. The relationships between the number of pollen grains and the frequencies of ovules with zero, one and two pollen tubes. Wild-type pistils were pollinated by hap2-1/quartet mutant pollen grains. Blue bars indicate the percentage of ovules without a pollen tube, light-green bars indicate the percentage of ovules with one pollen tube and pink bars indicate the percentage of ovules with two pollen tubes. Two days after pollination, under the restricted condition (20 and 40 pollen grains; ovules > pollen tubes), most ovules accepted either zero or one pollen tube. Under the saturated condition (80, 120 and 700 grains; ovules < pollen grains), most ovules accepted one or two pollen tube(s).
Acknowledgments
We thank N. Iwata for assistance in preparing plant materials. We thank Y. Ono (JEOL Ltd.), T. Suzuki and N. Sasaki for SEM analysis. D.M. was supported by GCOE program (Nagoya University) and grant number 6526 from the Japan Society for the Promotion of Science Fellowships. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (numbers 18075004 and 19370017 to T.H.), the Japan Science and Technology Agency (PRESTO project to T.H.), the Yamada Science Foundation (to T.H.) and the Mitsubishi Foundation (to T.H.) and the Biotechnology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Wylie RB. The morphology of Elodea canadensis. Contributions from the Hull Botanical Laboratory. LII. Bot Gaz 1904; 37:1 - 22; http://dx.doi.org/10.1086/328440
- Maheshwari P. An Introduction to the Embryology of Angiosperms McGraw-Hill, New York, 1950.
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 2006; 8:64 - 71; http://dx.doi.org/10.1038/ncb1345; PMID: 16378100
- von Besser K, Frank AC, Johnson MA, Preuss D. ArabidopsisHAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 2006; 133:4761 - 9; http://dx.doi.org/10.1242/dev.02683; PMID: 17079265
- Twell D, Wing R, Yamaguchi J, McCormick S. Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet 1989; 217:240 - 5; http://dx.doi.org/10.1007/BF02464887; PMID: 2770694
- Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, et al. Arabidopsis hapless mutations define essential gametophytic functions. Genetics 2004; 168:971 - 82; http://dx.doi.org/10.1534/genetics.104.029447; PMID: 15514068
- Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol 2012; 22:1084 - 9; http://dx.doi.org/10.1016/j.cub.2012.03.069; PMID: 22608509
- Beale KM, Leydon AR, Johnson MA. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr Biol 2012; 22:1090 - 4; http://dx.doi.org/10.1016/j.cub.2012.04.041; PMID: 22608506
- Rotman N, Durbarry A, Wardle A, Yang WC, Chaboud A, Faure JE, et al. A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol 2005; 15:244 - 8; http://dx.doi.org/10.1016/j.cub.2005.01.013; PMID: 15694308
- Brownfield L, Hafidh S, Durbarry A, Khatab H, Sidorova A, Doerner P, et al. Arabidopsis DUO POLLEN3 is a key regulator of male germline development and embryogenesis. Plant Cell 2009; 21:1940 - 56; http://dx.doi.org/10.1105/tpc.109.066373; PMID: 19638475
- Preuss D, Rhee SY, Davis RW. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 1994; 264:1458 - 60; http://dx.doi.org/10.1126/science.8197459; PMID: 8197459
- Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, et al. Pollen tube attraction by the synergid cell. Science 2001; 293:1480 - 3; http://dx.doi.org/10.1126/science.1062429; PMID: 11520985
- Fernando DD, Quinn CR, Brenner ED, Owens JN. Male gametophyte development and evolution in extant gymnosperms. Int J Plant Dev Biol 2010; 4:47 - 63
- Friedman WE. Embryological evidence for developmental lability during early angiosperm evolution. Nature 2006; 441:337 - 40; http://dx.doi.org/10.1038/nature04690; PMID: 16710419
- Williams JH. Amborella trichopoda (Amborellaceae) and the evolutionary developmental origins of the angiosperm progamic phase. Am J Bot 2009; 96:144 - 65; http://dx.doi.org/10.3732/ajb.0800070; PMID: 21628181