Abstract
Plants depend on light during all phases of its life cycle, and have evolved a complex signaling network to constantly monitor its surroundings. Photomorphogenesis, a process during which the plant reprograms itself in order to dwell life in presence of light is one of the most studied phenomena in plants. Recent mutant analyses using model plant Arabidopsis thaliana and protein interaction assays have unraveled a new set of players, an 8-member subfamily of B-box proteins, known as BBX subfamily IV. For the members of this subfamily, positive (BBX21, BBX22) as well as negative (BBX24) functions have been described for its members, showing a strong association to two major players of the photomorphogenic cascade, HY5 and COP1. The roles of these new BBX regulators are not restricted to photomorphogenesis, but also have functions in other facets of light-dependent development. Therefore this newly identified set of regulators has opened up new insights into the understanding of the fine-tuning of this complex process.
Introduction
Plants, as autotrophs, depend on light for their survival. Light influences seed germination, gravitropism, seedling de-etiolation, circadian rhythms and flowering time.Citation1 One of the most important processes during plant development is photomorphogenesis, which involves transition from a heterotrophic darkness-dwelling state (skotomorphogenesis) to an autotrophic light-dwelling state (photomorphogenesis). The morphological changes accompanying this transition include the inhibition of hypocotyl elongation, opening of cotyledons and chlorophyll and anthocyanin biosynthesis.Citation2 In Arabidopsis, this process is governed by the interplay of the different photoreceptors. These receptors include the red/far-red absorbing phytochromes (PhyA- PhyE), the blue/UV-A absorbing cryptochromes (Cry1, Cry2, Cry3), phototropins (Phot1, Phot2), and the recently identified UV-B photoreceptor UVR8.Citation3 Phytochromes A and B regulate the major responses to red and far red light such as seedling de-etiolation, root and hypocotyl growth, entrainment of the circadian clock, repression of flowering and regulation of shade avoidance, among many others.Citation4-Citation11 PHYA is the predominant phytochrome in etiolated seedlings, and it is rapidly degraded upon PHYAFR photoconversion.Citation12 PHYB is the predominant photoreceptor during prolonged red light responses in plants.Citation5,Citation13,Citation14 The cryptochrome photoreceptors Cry1 and Cry2 dominate general de-etiolation responses under blue light; similarly, they are involved in flowering control, and the resetting of the circadian clock is partially dependent on the activity of this protein family.Citation15-Citation17 In darkness, the photomorphogenic process is negatively regulated by the COP/DET/FUS repressors.Citation18,Citation19 The most remarkable characteristic of the COP/DET/FUS-mutants is the display of photomorphogenic development in the dark. The group can be divided into three main complexes, according to their interactions: the COP10-DDB1-DET1 (CDD) complex, the COP9 signalosome (CSN) and the COP1 complex.Citation20-Citation23
Research efforts have been made to identify the signaling networks downstream the initial perception of the light stimulus.Citation24-Citation30 The photomorphogenic process is regulated by a multi-member gene network, in which the joint action of individual photoreceptors triggers the responses of the seedling to light adaptation. This process occurs at most levels of gene regulation, from chromatin conformational changes to protein modification and regulation.Citation24,Citation31,Citation32 Among the signaling regulators described, special attention has been drawn to the transcriptional regulators. The main downstream effectors of the photomorphogenesis cascade can be grouped in bZIP,Citation33-Citation35 bHLH,Citation26,Citation36 MYB,Citation37 Mutator-like transposasesCitation38 and the recently described B-box Zinc-finger proteins, named the BBX protein family.Citation39,Citation40
The B-box zinc finger protein family (BBX familyCitation40) has been implicated in the regulation of light-influenced processes, such as photomorphogenesis, shade avoidance, circadian rhythm and flowering. Its most well-known member, CONSTANS (CO, BBX1) is an important regulator of flowering in the photoperiod pathway.Citation41,Citation42 BBX proteins present a modular arrangement of one or two B-box zinc finger motifs in its N-terminal region. The family was organized into five sub-families. This review will document the described functional roles and mechanisms of regulation for each member of the BBX subfamily IV and give some insights on the probable relation of the members to the major regulators of photomorphogenesis and light signaling.
BBX Protein Classification
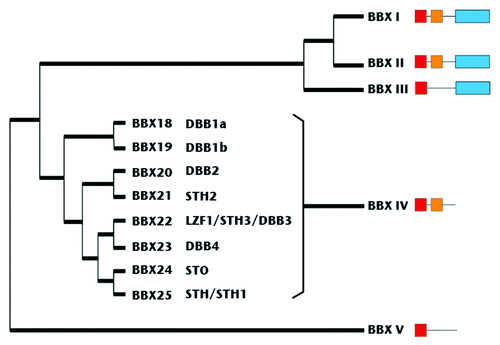
The BBX protein family is divided into five main clades according to its protein sequence.Citation40 Sub-family I consist of six members (including CO; BBX1−6); sub-family II has seven members (BBX7−13). Both sub-families share the same structure: two B-box zinc finger motifs at the N-terminus and a CCT (CONSTANS CONSTANS-LIKE TOC1) domain at the C-terminus (BBX1-13) (). Sub-family III is formed by four members (BBX14−17) that share one B-box motif and also a CCT domain. Sub-family IV has eight members and carries only two B-box zinc fingers (motifs) (BBX18−25), and the final group (Sub-family V) has one B-box motif and is composed of 7 members (BBX26−32).
Figure 1. Phylogenetic tree of BBX family modified from Khanna et al.40 The red and yellow boxes represent B-boxes type 1 and 2, respectively.40,52 The blue box represents the CCT (CO COL TOC) domain.
The C-terminal CCT (CO-COL-TOC1) domain is believed to modulate protein and DNA binding.Citation43 The B-box domains in the whole family are thought to mediate protein-protein interactions, but confirmation of these interactions is described only in few individuals.Citation44-Citation46 Functional description of other members of this family have uncovered roles in circadian rhythm regulation for COL1,Citation47 red light photomorphogenic signaling for COL3,Citation48 flowering control for COL5 and COL9Citation49,Citation50 and negative control of light-induced genes in the dark for BBX32.Citation51
Sub-family IV is composed of eight members (). Protein alignments show around 20% amino acid identity among the members, but close homologs are observed in BBX18−BBX19 and BBX24−BBX25 protein pairs with 70% of amino acid identity for each pair. As noted before, all members share around 55% amino acid identity in the B-box domains.Citation40,Citation44,Citation45,Citation52
Function and Regulation
BBX24 and BBX25
SALT TOLERANCE (STO/BBX24) was the first member of the sub-family which was linked to light signaling. BBX24 was initially described as a protein which could complement the salt sensitive phenotype of yeast mutants.Citation53,Citation54 Nagaoka and Takano (2003) showed that Arabidopsis plants overexpressing BBX24 had an enhanced tolerance to salty media.Citation55
BBX24 interacts in yeast-two-hybrid assays with the hormone and abiotic stress regulator CLONE EIGHTY-ONE/ RADICAL INDUCED CELL DEATH1 (CEO/RCD1)Citation56,Citation57 and with COP1, a negative regulator of photomorphogenesis.Citation58 Holm and colleagues (2001) described a conserved amino-acid motif shared by BBX24, BBX25 and HY5 (V-P-E/D-Φ-G; Φ = hydrophobic residue) which is needed for a correct interaction with the WD40 domain of COP1.
BBX24 was linked to the photomorphogenic signaling cascade as a putative negative regulator ().Citation39,Citation59 BBX24 gain- and loss-of-function mutants displayed contrasting hypocotyl elongation and reciprocal cotyledon expansion when grown under different qualities of monochromatic light.Citation59 Furthermore, Indorf and collaborators showed that the BBX24 protein is nuclear-localized, co-localizes with COP1 in the nucleus, and is degraded by a COP1-dependent mechanism. Most interesting, BBX24 accumulation in planta presented light-dependent stabilization, which makes of BBX24 a negative regulator of photomorphogenesis during the light stimulus.
Table 1. Identification and Biological functions of BBX sub-family IV members
Yan et al.Citation60 reported that interaction with COP1 is required for proper function and protein turnover of BBX24 in light. Interestingly, transgenic plants overexpressing BBX24 with a mutation in the COP1-interacting motif (bbx24COP1) did not accumulate the protein in darkness, where COP1 is supposed to be active. Genetic analyses suggest that although COP1 needs to be present, no direct interaction with COP1 is required for protein degradation in darkness.Citation60 Protein analyses in yeast demonstrated that this mutation did not provoke structural defects in the protein. An interesting observation described by Yan et al.Citation60 is the BBX24-mediated transcriptional activation when fused to a DNA-Binding domain in yeast 2-hybrid experiments. This result hints a role of BBX24 in transcriptional activation. A recent report links BBX24 to UV-B dependent signaling pathways.Citation61 The authors demonstrate that the bbx24 mutant displays a hypersensitive phenotype under low intensity UV-B. Furthermore, BBX24 interacts with COP1 upon UV-B induction and antagonizes HY5 UV-B induced inhibition of hypocotyl elongation by suppression of its transcriptional activity.Citation61
The homolog of BBX24, BBX25, has been reported to function as a negative regulator of hypocotyl elongation under red and far-red light.Citation39,Citation62 The phenotype described for bbx25 mutants is weak, when compared with bbx24 alleles. Given the homology presented between this protein pair, it can be argued that the presence of a functional BBX24 protein in the bbx25 mutant background can conceal a stronger effect probably due to redundancy, as observed already for protein homologs in the light signaling cascade.Citation33,Citation63,Citation64 Both homologs might have related but independent functions during photomorphogenesis, being BBX24 the major player of the pair, and BBX25 having a minor role during photomorphogenesis (unpublished results, F. Sarmiento). The report by Jiang and colleagues (2012) of BBX24 involved in UV-B signaling opens another functional window for possible functions of BBX25 in this developmental cascade.
BBX18 and BBX19
BBX18 and BBX19 protein pair were initially characterized as putative negative regulators of photomorphogenesis ().Citation39 The expression of both transcripts is regulated by the circadian rhythm, and 35S:BBX18 and 35S:BBX19 overexpression plants display hyposensitivity to red and far-red light. Experiments by Wang and colleaguesCitation65 demonstrated that BBX18 is a nuclear-localized protein. Light fluence-response analyses of BBX18 gain-and-loss-of-function lines proved that this gene is involved in blue light-dependent hypocotyl elongation as a negative regulator, and its phenotype is related to gibberellin signaling pathways.Citation65 Further experiments have proved that BBX18 is involved as a negative regulator of basal and acquired thermotolerance.Citation66 In addition, BBX18 has been related to flower development.Citation67
BBX21 and BBX22
STH2/BBX21 and STH3/LZF1/BBX22, even though they are not close homologs (),Citation40 have striking functional similarities. Both proteins interact in yeast and plants with HY5.Citation44,Citation45 The B-box motifs of both proteins are important for the interaction with HY5 and have been characterized as positive regulators of light signaling.Citation44,Citation45,Citation52 BBX22 interacts with HY5 HOMOLOG (HYH)Citation44 and its expression in light depends on a functional HY5.Citation52 Knockout T-DNA alleles of STH2/BBX21 and STH3/ LZF1/BBX22 (sth2–1, lzf1–1) display a hyposensitive phenotype under different light qualities, reduced anthocyanin level, and are involved in HY5-dependent and independent signaling.Citation44,Citation45,Citation52
Furthermore the genetic interaction with COP1 also depicted similarities between BBX21 and BBX22. Allele-specific complementation of cop1 mutation effects (hypocotyl growth, anthocyanin accumulation) was observed in genetic interactions of bbx22–1 with cop1–4 and cop1–6.Citation44 On the other hand, the absence of BBX21 in the cop1–4 and cop1–6 alleles partially suppressed hypocotyl reduction and anthocyanin accumulation displayed by these mutants.Citation45 The joint effect of bbx21–1 and lzf1–1/sth3 in the cop1–4 and cop1–6 backgrounds strongly suppressed hypocotyl inhibition of the cop1 alleles and reduced anthocyanin accumulation in the cop1–4 and cop1–6 alleles, respectively.Citation44 This suggests that the misregulation of these BBX proteins is an important event for the phenotypes displayed by the cop1 alleles.Citation44
Despite these described genetic interactions, there was no physical interaction detected between COP1 and BBX21 or BBX22 in plant cells, although co-localization to the same sub-nuclear bodies was observed.Citation44,Citation45 In vitro ubiquitination of BBX22 in the presence of COP1 was also reported.
The similarity in the phenotypes of the loss-of-function mutants and the genetic interaction with HY5 implies that these two proteins act redundantly in the regulation of photomorphogenesis. Indeed, phenotypic characterization of the sth2–1sth3/lzf1–1 double mutant demonstrated the enhanced phenotypes observed in hypocotyl growth and anthocyanin accumulation.Citation44 Transcription activation assays in protoplasts demonstrated that BBX21 and BBX22 were able to activate the transcription of chlorophyll a/b binding protein (CAB) and chalcone isomerase (CHI).Citation44,Citation52 The joint action of both proteins greatly increased transcription efficiency of the luciferase gene fused to the promoters of CAB and CHI.Citation44,Citation52 Mutations in the proposed G-box of the tested promoters or in the B-boxes diminished the transcriptional activation ability. Interestingly, these experiments also uncovered a higher affinity of BBX21 for the CHI promoter and BBX22 for the CAB promoter.
The reports by Datta and colleaguesCitation44,Citation45 and Chang et al.Citation52 also described specific roles for each of the studied BBX proteins (). A close examination of the sth2–1/bbx21–1 allele revealed an increased number of emerged lateral roots compared with the WT when the plants were grown in short days (SD). Genetic interaction analyses in this condition uncovered that the hy5–215 allele was epistatic to bbx21–1.Citation45 Additionally the lzf1–1/bbx22–1 mutant displayed a lower amount of chlorophyll and a delay in the formation of chloroplasts in etiolated plants treated with white light.Citation52
Chang and colleaguesCitation68 demonstrated that BBX22, as previously described for BBX24, is tightly regulated at a post-translational level, and this control is mediated by HY5 and COP1. BBX22-GFP Protein analyses in the hy5–1 and cop1–4 mutants revealed that COP1 is required for protein degradation of BBX22 in the dark, while HY5 has a role in the degradation of BBX22 in light.Citation68 Consistent with these observations, phenotypic analyses of 35S:BBX22-GFPox-cop1 lines proved that the overexpression of BBX22-GFP enhanced the cop1 phenotype during skotomorphogenesis and photomorphogenesis. In addition, growth rate experiments for the bbx22 line showed that under short days the mutant displayed an increased growth rate compared with WT during the dark phase in day 4 after germination. This suggests a more prominent role for BBX22 in SD adaptive processes, by possibly transmitting the signal for attenuated hypocotyl elongation during the night.Citation68
A recent report links the BBX protein sub-family IV to the shade avoidance syndrome (SAS).Citation69 A T-DNA mutant screening for seedlings with longer hypocotyls under simulated shade, but with WT phenotype under white light and dark conditions, isolated a new bbx21–101 allele which displayed a longer hypocotyl than the WT when grown under shade. The mutant alleles bbx19, bbx21 and bbx22 displayed an enhanced hypocotyl elongation, while bbx18 and bbx24 exhibited the opposite phenotype under shade, hinting a function during the shade avoidance response. In contrast, no phenotype was observed for the bbx25 allele.Citation69 The authors conclude that BBX21 is a negative regulator of SAS in conjunction with specific hormone-mediated signaling cascades. BBX21 promotes the expression of genes early responding to simulated shade, and represses some genes under long-term canopy shade. Furthermore, genetic and expression analyses suggest that BBX21 and BBX22 are involved in COP1-mediated regulation of the shade avoidance response.Citation69
BBX20 and BBX23
BBX20 and BBX23 seem to be elusive members of this sub-family (). Expression analyses were performed under continuous light by Kumagai and colleagues,Citation39 but the transcripts of these two genes were not detected. A report describing transcriptional responses to karrikins, a germination stimulant identified in the smoke of wildfires, characterized BBX20/STH7 as an early response transcript.Citation70 The transcriptional response of BBX20/STH7 to karrikins during germination was independent of light, gibberellins and light regulator HY5. However, it is still not known if BBX20 has a role in karrikin-dependent germination responses. Fan et al.Citation71 described the function of BBX20 as a negative regulator of brassinosteroid (BR) signaling, but a positive regulator of photomorphogenesis. The authors found that BBX20 is regulated transcriptionally via BR signaling (by BZR1), and posttranscriptionally by means of a COP1-dependent mechanism,Citation71 similarly to GATA2 regulation.Citation72 Confocal microscopy analyses localize BBX20:YFP in the nucleolus and cytoplasm, making this feature unique for this protein in sub-family IV.Citation71 Much less is known for BBX23. Genevestigator transcriptomic data mining showed that the condition under which the BBX23 transcript is upregulated is darkness.Citation73
The Role of Subfamily IV BBX Proteins
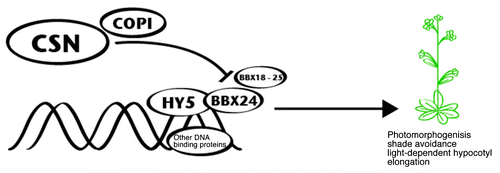
Most of the data here presented point to the fact that the BBX sub-family IV might be transcriptional regulators strongly linked to the light signaling cascade, especially associated with COP1 and HY5 regulators, and probably cross-talk with other networks, such as different hormonal pathways.Citation65,Citation71 Independent experiments demonstrate that BBX21, BBX22 and BBX24 have trans-activating capacity in transcriptional systems,Citation44,Citation45,Citation51,Citation60 although no DNA binding ability has been described. The proposed mechanism of action uses the interaction with other transcription factors that bind to DNA, such as HY5 and HYH ().Citation44,Citation45 It has been documented that HY5 is able to bind to promoters of genes that apparently are not directly activated by this transcription factor;Citation74 therefore it is probable that HY5 acts in combination with other transcriptional activators, such as the BBX proteins, as was previously proposed.Citation45 Given that there are positive and negative regulators of photomorphogenesis in this group, a fine tuning of photomorphogenesis by differential binding of these BBX proteins to HY5 and maybe other transcriptional activators might occur (). Although interaction with HY5 has been described in yeast 2-hybrid assays, there is only indirect evidence for the HY5-BBX complexes in plants. Datta and colleagues,Citation45 were unable to detect the complex formation in-vitro, probably due to lack of other co-factors.
Figure 2. Model of BBX24 and BBX sub-family IV proteins mode of action and regulation. As potential transcriptional activators,60,68 BBX24 and its close relatives probably require a DNA binding protein, such as HY5 to fulfill its function.44,45 Once bound to the transcriptional complex, these BBX proteins will activate transcription in order to regulate diverse processes, such as photomorphogenesis, shade avoidance and light-dependent hypocotyl elongation. Evidence points to a COP1-mediated protein regulation of BBX24 and probably other BBX proteins from sub-family IV.59, 60 Experiments with cop1 alleles demonstrated that COP1 is required for correct protein turnover of BBX24 and BBX22.60, 68
Holtan et al.Citation51 described the formation of heterodimers between BBX21 and BBX32, and probably homodimers of BBX21, by means of its B-box motifs. The authors suggest a mechanism of regulation in which BBX32 binds to BBX21 to modulate its action in plants. It might be possible, that besides a possible differential binding to HY5, there might be also co-regulation of these factors by heterodimer formation. Efforts must be set into the analysis of these interactions. Up to date, there are no reports on the interactions among members of this BBX sub-family, except for the similar roles of BBX21 and BBX22, and there are members still with unknown function (BBX23). Construction of higher order mutants, protein interaction assays, co-immune precipitations and transcriptional activation assays could elucidate the mode of action, similarities and differences of the members of the BBX sub-family IV proteins.
A close relationship has been proposed between these BBX proteins and signaling regulator COP1. The main function of COP1 is targeting positive regulators of photomorphogenesis and regulation of flowering in the photoperiod pathway,Citation75-Citation77 all functions related to its E-3 ubiquitin-ligase activity in the dark. The latest reports have attributed roles for COP1 in light, during the first hours of de-etiolation,Citation60,Citation78 and a recent model explains the protein kinetics of two known COP1 targets during de-etiolation as part of a molecular switch involving two ubiquitin-ligase complexes.Citation79 COP1 is probably targeting the BBX proteins for degradation as it does for CO/BBX1 ().Citation76,Citation77 This theory was tested by Yan et al.,Citation60 and their experiments suggest that the previously described interaction of BBX24 with COP1 is needed for the correct protein turnover of BBX24 and also for the function of BBX24 in plants.
Even though ubiquitination of BBX22 by COP1 was described in vitro, no direct interaction was observed in vivo.Citation44 Direct interaction with COP1 is not needed for COP1-mediated protein regulation, as was reported for GIGANTEA protein degradation.Citation75 In this line of thought, it is possible that BBX22 and also other BBX proteins are targeted for COP1-mediated protein degradation by means of adaptor proteins. Evidence on BBX24 and BBX22 points out that post-translational regulation might be the most important regulatory step for the BBX proteins. Saijo et al.Citation80 detected mono-ubiquitinated phyA in in vitro assays, suggesting that COP1-mediated ubiquitination might not only serve to target proteins for degradation. Whether COP1 or other ubiquitination machinery is in charge of controlling the action of any of these proteins still has to be elucidated.
Since the first report on STO/BBX24Citation54 there have been advances in the description of this subfamily; a radical shift was made from a possible function in salt stress to a role in light signaling. Reports on the other members of this subfamily have uncovered various light-related functions and have linked these proteins to important regulators of light signaling such as COP1 and HY5 and to hormone pathways. Further research is needed to define how the BBX proteins interact with each other and with common partners in order to fine-tune light-related responses.
Acknowledgments
I wish to thank Alejandra Sarmiento for the help provided with the diagrams, and Mauricio Quimbaya, Cornelia Bär, Jacobo Arango, Teresa Mosquera and the members of the Neuhaus lab at the University of Freiburg for all the useful discussions and comments before and during the completion of this manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Casal JJ, Luccioni LG, Oliverio KA, Boccalandro HE. Light, phytochrome signalling and photomorphogenesis in Arabidopsis. Photochem Photobiol Sci 2003; 2:625 - 36; http://dx.doi.org/10.1039/b300094j; PMID: 12859146
- Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol 1998; 118:27 - 35; http://dx.doi.org/10.1104/pp.118.1.27; PMID: 9733523
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011; 332:103 - 6; http://dx.doi.org/10.1126/science.1200660; PMID: 21454788
- Franklin KA, Larner VS, Whitelam GC. The signal transducing photoreceptors of plants. Int J Dev Biol 2005; 49:653 - 64; http://dx.doi.org/10.1387/ijdb.051989kf; PMID: 16096972
- Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot 2010; 61:11 - 24; http://dx.doi.org/10.1093/jxb/erp304; PMID: 19815685
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, et al. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 1993; 5:757 - 68; PMID: 8364355
- Guo H, Yang H, Mockler TC, Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science 1998; 279:1360 - 3; http://dx.doi.org/10.1126/science.279.5355.1360; PMID: 9478898
- Hennig L, Poppe C, Unger S, Schäfer E. Control of hypocotyl elongation in Arabidopsis thaliana by photoreceptor interaction. Planta 1999; 208:257 - 63; http://dx.doi.org/10.1007/s004250050557; PMID: 10333589
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. The hy3 Long Hypocotyl Mutant of Arabidopsis Is Deficient in Phytochrome B. Plant Cell 1991; 3:1263 - 74; PMID: 12324590
- Franklin KA, Whitelam GC. Phytochrome a function in red light sensing. Plant Signal Behav 2007; 2:383 - 5; http://dx.doi.org/10.4161/psb.2.5.4261; PMID: 19704607
- Franklin KA. Shade avoidance. New Phytol 2008; 179:930 - 44; http://dx.doi.org/10.1111/j.1469-8137.2008.02507.x; PMID: 18537892
- Clough RC, Vierstra RD. Phytochrome degradation. Plant Cell Environ 1997; 20:713 - 21; http://dx.doi.org/10.1046/j.1365-3040.1997.d01-107.x
- Rausenberger J, Hussong A, Kircher S, Kirchenbauer D, Timmer J, Nagy F, et al. An integrative model for phytochrome B mediated photomorphogenesis: from protein dynamics to physiology. PLoS One 2010; 5:e10721; http://dx.doi.org/10.1371/journal.pone.0010721; PMID: 20502669
- Hu W, Su YS, Lagarias JC. A light-independent allele of phytochrome B faithfully recapitulates photomorphogenic transcriptional networks. Mol Plant 2009; 2:166 - 82; http://dx.doi.org/10.1093/mp/ssn086; PMID: 19529817
- El-Din El-Assal S, Alonso-Blanco C, Peeters AJ, Wagemaker C, Weller JL, Koornneef M. The role of cryptochrome 2 in flowering in Arabidopsis. Plant Physiol 2003; 133:1504 - 16; http://dx.doi.org/10.1104/pp.103.029819; PMID: 14605222
- Yanovsky MJ, Mazzella MA, Whitelam GC, Casal JJ. Resetting of the circadian clock by phytochromes and cryptochromes in Arabidopsis. J Biol Rhythms 2001; 16:523 - 30; http://dx.doi.org/10.1177/074873001129002213; PMID: 11760010
- Lin C, Shalitin D. Cryptochrome structure and signal transduction. Annu Rev Plant Biol 2003; 54:469 - 96; http://dx.doi.org/10.1146/annurev.arplant.54.110901.160901; PMID: 14503000
- Schwechheimer C, Deng XW. The COP/DET/FUS proteins-regulators of eukaryotic growth and development. Semin Cell Dev Biol 2000; 11:495 - 503; http://dx.doi.org/10.1006/scdb.2000.0203; PMID: 11145879
- Kwok SF, Piekos B, Misera S, Deng XW. A complement of ten essential and pleiotropic arabidopsis COP/DET/FUS genes is necessary for repression of photomorphogenesis in darkness. Plant Physiol 1996; 110:731 - 42; http://dx.doi.org/10.1104/pp.110.3.731; PMID: 8819865
- Schroeder DF, Gahrtz M, Maxwell BB, Cook RK, Kan JM, Alonso JM, et al. De-etiolated 1 and damaged DNA binding protein 1 interact to regulate Arabidopsis photomorphogenesis. Curr Biol 2002; 12:1462 - 72; http://dx.doi.org/10.1016/S0960-9822(02)01106-5; PMID: 12225661
- Peng Z, Serino G, Deng XW. A role of Arabidopsis COP9 signalosome in multifaceted developmental processes revealed by the characterization of its subunit 3. Development 2001; 128:4277 - 88; PMID: 11684663
- Chamovitz DA, Wei N, Osterlund MT, von Arnim AG, Staub JM, Matsui M, et al. The COP9 complex, a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 1996; 86:115 - 21; http://dx.doi.org/10.1016/S0092-8674(00)80082-3; PMID: 8689678
- Zhu D, Maier A, Lee JH, Laubinger S, Saijo Y, Wang H, et al. Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell 2008; 20:2307 - 23; http://dx.doi.org/10.1105/tpc.107.056580; PMID: 18812498
- Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet 2007; 8:217 - 30; http://dx.doi.org/10.1038/nrg2049; PMID: 17304247
- Chory J. Light signal transduction: an infinite spectrum of possibilities. Plant J 2010; 61:982 - 91; http://dx.doi.org/10.1111/j.1365-313X.2009.04105.x; PMID: 20409272
- Castillon A, Shen H, Huq E. Phytochrome Interacting Factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci 2007; 12:514 - 21; http://dx.doi.org/10.1016/j.tplants.2007.10.001; PMID: 17933576
- Fankhauser C, Chory J. Light control of plant development. Annu Rev Cell Dev Biol 1997; 13:203 - 29; http://dx.doi.org/10.1146/annurev.cellbio.13.1.203; PMID: 9442873
- Quail PH. Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol 2002; 3:85 - 93; http://dx.doi.org/10.1038/nrm728; PMID: 11836510
- Von Arnim A, Deng XW. Light Control of Seedling Development. Annu Rev Plant Physiol Plant Mol Biol 1996; 47:215 - 43; http://dx.doi.org/10.1146/annurev.arplant.47.1.215; PMID: 15012288
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Genet 2004; 38:87 - 117; http://dx.doi.org/10.1146/annurev.genet.38.072902.092259; PMID: 15568973
- Charron JB, He H, Elling AA, Deng XW. Dynamic landscapes of four histone modifications during deetiolation in Arabidopsis. Plant Cell 2009; 21:3732 - 48; http://dx.doi.org/10.1105/tpc.109.066845; PMID: 20008096
- Chen H, Huang X, Gusmaroli G, Terzaghi W, Lau OS, Yanagawa Y, et al. Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 2010; 22:108 - 23; http://dx.doi.org/10.1105/tpc.109.065490; PMID: 20061554
- Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 2002; 16:1247 - 59; http://dx.doi.org/10.1101/gad.969702; PMID: 12023303
- Desai M, Hu J. Light induces peroxisome proliferation in Arabidopsis seedlings through the photoreceptor phytochrome A, the transcription factor HY5 HOMOLOG, and the peroxisomal protein PEROXIN11b. Plant Physiol 2008; 146:1117 - 27; http://dx.doi.org/10.1104/pp.107.113555; PMID: 18203870
- Mallappa C, Singh A, Ram H, Chattopadhyay S. GBF1, a transcription factor of blue light signaling in Arabidopsis, is degraded in the dark by a proteasome-mediated pathway independent of COP1 and SPA1. J Biol Chem 2008; 283:35772 - 82; http://dx.doi.org/10.1074/jbc.M803437200; PMID: 18930926
- Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 2011; 16:19 - 28; http://dx.doi.org/10.1016/j.tplants.2010.08.003; PMID: 20833098
- Ballesteros ML, Bolle C, Lois LM, Moore JM, Vielle-Calzada JP, Grossniklaus U, et al. LAF1, a MYB transcription activator for phytochrome A signaling. Genes Dev 2001; 15:2613 - 25; http://dx.doi.org/10.1101/gad.915001; PMID: 11581165
- Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 2007; 318:1302 - 5; http://dx.doi.org/10.1126/science.1146281; PMID: 18033885
- Kumagai T, Ito S, Nakamichi N, Niwa Y, Murakami M, Yamashino T, et al. The common function of a novel subfamily of B-Box zinc finger proteins with reference to circadian-associated events in Arabidopsis thaliana. Biosci Biotechnol Biochem 2008; 72:1539 - 49; http://dx.doi.org/10.1271/bbb.80041; PMID: 18540109
- Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, Mizuno T, et al. The Arabidopsis B-box zinc finger family. Plant Cell 2009; 21:3416 - 20; http://dx.doi.org/10.1105/tpc.109.069088; PMID: 19920209
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995; 80:847 - 57; http://dx.doi.org/10.1016/0092-8674(95)90288-0; PMID: 7697715
- Onouchi H, Igeño MI, Périlleux C, Graves K, Coupland G. Mutagenesis of plants overexpressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. Plant Cell 2000; 12:885 - 900; PMID: 10852935
- Tiwari SB, Shen Y, Chang HC, Hou Y, Harris A, Ma SF, et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol 2010; 187:57 - 66; http://dx.doi.org/10.1111/j.1469-8137.2010.03251.x; PMID: 20406410
- Datta S, Johansson H, Hettiarachchi C, Irigoyen ML, Desai M, Rubio V, et al. LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 2008; 20:2324 - 38; http://dx.doi.org/10.1105/tpc.108.061747; PMID: 18796637
- Datta S, Hettiarachchi C, Johansson H, Holm M. SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 2007; 19:3242 - 55; http://dx.doi.org/10.1105/tpc.107.054791; PMID: 17965270
- Robson F, Costa MM, Hepworth SR, Vizir I, Piñeiro M, Reeves PH, et al. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 2001; 28:619 - 31; http://dx.doi.org/10.1046/j.1365-313x.2001.01163.x; PMID: 11851908
- Ledger S, Strayer C, Ashton F, Kay SA, Putterill J. Analysis of the function of two circadian-regulated CONSTANS-LIKE genes. Plant J 2001; 26:15 - 22; http://dx.doi.org/10.1046/j.1365-313x.2001.01003.x; PMID: 11359606
- Datta S, Hettiarachchi GH, Deng XW, Holm M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 2006; 18:70 - 84; http://dx.doi.org/10.1105/tpc.105.038182; PMID: 16339850
- Hassidim M, Harir Y, Yakir E, Kron I, Green RM. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 2009; 230:481 - 91; http://dx.doi.org/10.1007/s00425-009-0958-7; PMID: 19504268
- Cheng XF, Wang ZY. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J 2005; 43:758 - 68; http://dx.doi.org/10.1111/j.1365-313X.2005.02491.x; PMID: 16115071
- Holtan HE, Bandong S, Marion CM, Adam L, Tiwari S, Shen Y, et al. BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol 2011; 156:2109 - 23; http://dx.doi.org/10.1104/pp.111.177139; PMID: 21632973
- Chang CS, Li YH, Chen LT, Chen WC, Hsieh WP, Shin J, et al. LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J 2008; 54:205 - 19; http://dx.doi.org/10.1111/j.1365-313X.2008.03401.x; PMID: 18182030
- Rodriguez-Franco M, Sarmiento F, Marquardt K, Markus R, Neuhaus G. Does light taste salty?. Plant Signal Behav 2008; 3:72 - 3; http://dx.doi.org/10.4161/psb.3.1.4925; PMID: 19704777
- Lippuner V, Cyert MS, Gasser CS. Two classes of plant cDNA clones differentially complement yeast calcineurin mutants and increase salt tolerance of wild-type yeast. J Biol Chem 1996; 271:12859 - 66; http://dx.doi.org/10.1074/jbc.271.22.12859; PMID: 8662738
- Nagaoka S, Takano T. Salt tolerance-related protein STO binds to a Myb transcription factor homologue and confers salt tolerance in Arabidopsis. J Exp Bot 2003; 54:2231 - 7; http://dx.doi.org/10.1093/jxb/erg241; PMID: 12909688
- Belles-Boix E, Babiychuk E, Van Montagu M, Inzé D, Kushnir S. CEO1, a new protein from Arabidopsis thaliana, protects yeast against oxidative damage. FEBS Lett 2000; 482:19 - 24; http://dx.doi.org/10.1016/S0014-5793(00)02016-0; PMID: 11018516
- Jaspers P, Blomster T, Brosché M, Salojärvi J, Ahlfors R, Vainonen JP, et al. Unequally redundant RCD1 and SRO1 mediate stress and developmental responses and interact with transcription factors. Plant J 2009; 60:268 - 79; http://dx.doi.org/10.1111/j.1365-313X.2009.03951.x; PMID: 19548978
- Holm M, Hardtke CS, Gaudet R, Deng XW. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J 2001; 20:118 - 27; http://dx.doi.org/10.1093/emboj/20.1.118; PMID: 11226162
- Indorf M, Cordero J, Neuhaus G, Rodríguez-Franco M. Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J 2007; 51:563 - 74; http://dx.doi.org/10.1111/j.1365-313X.2007.03162.x; PMID: 17605755
- Yan H, Marquardt K, Indorf M, Jutt D, Kircher S, Neuhaus G, et al. Nuclear localization and interaction with COP1 are required for STO/BBX24 function during photomorphogenesis. Plant Physiol 2011; 156:1772 - 82; http://dx.doi.org/10.1104/pp.111.180208; PMID: 21685177
- Jiang L, Wang Y, Li QF, Björn LO, He JX, Li SS. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. [Epub ahead of print] Cell Res 2012; 22:1046 - 57; http://dx.doi.org/10.1038/cr.2012.34; PMID: 22410790
- Khanna R, Shen Y, Toledo-Ortiz G, Kikis EA, Johannesson H, Hwang YS, et al. Functional profiling reveals that only a small number of phytochrome-regulated early-response genes in Arabidopsis are necessary for optimal deetiolation. Plant Cell 2006; 18:2157 - 71; http://dx.doi.org/10.1105/tpc.106.042200; PMID: 16891401
- Laubinger S, Fittinghoff K, Hoecker U. The SPA quartet: a family of WD-repeat proteins with a central role in suppression of photomorphogenesis in arabidopsis. Plant Cell 2004; 16:2293 - 306; http://dx.doi.org/10.1105/tpc.104.024216; PMID: 15308756
- Briggs GC, Osmont KS, Shindo C, Sibout R, Hardtke CS. Unequal genetic redundancies in Arabidopsis--a neglected phenomenon?. Trends Plant Sci 2006; 11:492 - 8; http://dx.doi.org/10.1016/j.tplants.2006.08.005; PMID: 16949326
- Wang Q, Zeng J, Deng K, Tu X, Zhao X, Tang D, et al. DBB1a, involved in gibberellin homeostasis, functions as a negative regulator of blue light-mediated hypocotyl elongation in Arabidopsis. Planta 2011; 233:13 - 23; http://dx.doi.org/10.1007/s00425-010-1274-y; PMID: 20872270
- Wang Q, Tu X, Zhang J, Chen X, Rao L. Heat stress-induced BBX18 negatively regulates the thermotolerance in Arabidopsis. Mol Biol Rep 2012; 14:14; PMID: 23238922
- Wang Q, Tu X, Deng K, Zeng J, Zhao X, Tang D, et al. A Defect in Zinc Finger Protein Double B-box 1a (DBB1a) Causes Abnormal Floral Development in Arabidopsis.. J Plant Biol 2009; 52:543 - 9; http://dx.doi.org/10.1007/s12374-009-9070-6
- Chang CS, Maloof JN, Wu SH. COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol 2011; 156:228 - 39; http://dx.doi.org/10.1104/pp.111.175042; PMID: 21427283
- Crocco CD, Holm M, Yanovsky MJ, Botto JF. AtBBX21 and COP1 genetically interact in the regulation of shade avoidance. Plant J 2010; 64:551 - 62; http://dx.doi.org/10.1111/j.1365-313X.2010.04360.x; PMID: 21070414
- Nelson DC, Flematti GR, Riseborough JA, Ghisalberti EL, Dixon KW, Smith SM. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc Natl Acad Sci USA 2010; 107:7095 - 100; http://dx.doi.org/10.1073/pnas.0911635107; PMID: 20351290
- Fan XY, Sun Y, Cao DM, Bai MY, Luo XM, Yang HJ, et al, XY F. BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol Plant 2012; 5:591 - 600; http://dx.doi.org/10.1093/mp/sss041; PMID: 22535582
- Luo XM, Lin WH, Zhu S, Zhu JY, Sun Y, Fan XY, et al. Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Dev Cell 2010; 19:872 - 83; http://dx.doi.org/10.1016/j.devcel.2010.10.023; PMID: 21145502
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008; 2008:1 - 420747; http://dx.doi.org/10.1155/2008/420747; PMID: 19956698
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, et al. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 2007; 19:731 - 49; http://dx.doi.org/10.1105/tpc.106.047688; PMID: 17337630
- Yu JW, Rubio V, Lee NY, Bai S, Lee SY, Kim SS, et al. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell 2008; 32:617 - 30; http://dx.doi.org/10.1016/j.molcel.2008.09.026; PMID: 19061637
- Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, et al. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 2008; 27:1277 - 88; http://dx.doi.org/10.1038/emboj.2008.68; PMID: 18388858
- Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, et al. COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 2008; 20:292 - 306; http://dx.doi.org/10.1105/tpc.107.057281; PMID: 18296627
- Jang IC, Henriques R, Seo HS, Nagatani A, Chua NH. Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 2010; 22:2370 - 83; http://dx.doi.org/10.1105/tpc.109.072520; PMID: 20605855
- Pokhilko A, Ramos JA, Holtan H, Maszle DR, Khanna R, Millar AJ. Ubiquitin ligase switch in plant photomorphogenesis: A hypothesis. J Theor Biol 2011; 270:31 - 41; http://dx.doi.org/10.1016/j.jtbi.2010.11.021; PMID: 21093457
- Saijo Y, Zhu D, Li J, Rubio V, Zhou Z, Shen Y, et al. Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol Cell 2008; 31:607 - 13; http://dx.doi.org/10.1016/j.molcel.2008.08.003; PMID: 18722184