Abstract
The role of microtubules in cellular pathways of UV-B signaling in plants as well as in related structural cell response become into focus of few last publications. As microtubules in plant cell reorient/reorganize (become randomized, fragmented or depolymerized) in a response to direct UV-B exposure, these cytoskeletal components could be involved into UV-B signaling pathways as highly responsive players. In the current addendum, indirect UV-B-induced microtubules reorganization in cells of shielded Arabidopsis thaliana (GFP-MAP4) primary roots and the correspondence of microtubules depolymerization with the typical hallmarks of the programmed cell death in Nicotiana tabacum BY-2 (GFP-MBD) cells are discussed.
Recently, the “paradigm shift” in understanding of cytoskeleton’s role in eukaryotic cell occurred. Apart from its “classical” functions such as cell division and growth, scaffolding, transport, microcompartmentation, etc,Citation1 the coordinated network of microtubules (MTs), actin filaments, microtubule-/actin-related proteins and others is definitely known to be intensely involved in cell signaling events (for review see refs. Citation2–Citation4). The organization and the dynamic properties of plant cytoskeleton are regulated by a broad range of intracellular signaling molecules, namely phytohormones,Citation3,Citation5,Citation6 reactive oxygen (ROS) and nitrogen species (RNS),Citation4,Citation7 Ca2+ 8,9, by protein kinase/phosphatase activitiesCitation10-Citation12 and others. Adaptive rearrangement of cytoskeleton in a response to both physiological environmental stimuli (gravity, touch, fluctuations of temperature, humidity, illumination regimes) and (a)biotic stress conditions (plant pathogens, toxic metals and herbicides pollution, salinization, high doses of UV irradiation) is determined by such intrinsic properties of cytoskeletal proteins as dynamic instability, threadmilling, bundling and abundant posttranslational modifications (for review see refs.Citation13–Citation16).
One of the constitutive abiotic environmental factors, UV non-ionising and mainly non-photosynthetically active radiation (UV, 200–400 nm)Citation17 challenges adaptive morphogenic responses in plantsCitation18 as well as a set of destructive effects.Citation19,Citation20 Its minor, but influential portion, UV B (UV-B, 280–315 nm) and, in a lesser extent, UV-A (315–400 nm) alters plant growth and morphology (lowering/increase of cell division rate, axillary branching, leaf thickening, cotyledon curling, number and/or diameter of inflorescence increase, root/shoot ratio shift, etc.).Citation18,Citation19 Cytoskeleton role in such UV-induced morphological changes as their driving force remains poorly investigated, since only a few articles are focused on the cytoskeleton reorganization in vitro as one of the events underlying UV-B-induced responses of plant cell.Citation21-Citation25 It was shown recently that the interphase and mitotic MTs in epidermal and cortex cells of all primary root zones of Arabidopsis thaliana L. seedlings expressing gfp-map4 (microtubule-assosiated protein 4) were randomized, depolymerized and/or stabilized in dose-dependent manner after the UV-B exposure (13.6–68 kJ/m2) in vivo that was accompanied by the cell swelling and excessive root hairs formation.Citation26 Our further experiments give additional evidences that plant MTs are involved in signal transduction under UV-B stress. The experimental system based on A. thaliana (GFP-MAP4) 4 d-old seedlings having their primary roots protected with aluminum foil downwards from the hypocotyls (hereinafter referred to as shielded seedlings) to avoid the direct UV-B exposure were designed. Thus, in 2 h after the UV-B irradiation (27.2 and 68 kJ/m2) of A. thaliana seedlings with shielded primary roots, randomization, depolymerization and/or bundling of MTs in epidermal cells of both shoots and roots occurred that was found in vivo by confocal microscopy ().
Figure 1. I. Cortical MTs organization in epidermal cells of A. thaliana in 2 h after the UV-B exposure: А − leaf, control; A' − leaf, 27.2 kJ/m2; A'' − leaf, 68 kJ/m2; B -hypocotyl, control; B' − hypocotyl, 27.2 kJ/m2; B'' − hypocotyl, 68 kJ/m2; C' − primary root transition zone, control; C' -primary root transition zone, 27.2 kJ/m2; C'' − primary root transition zone, 68 kJ/m2. Bar - 20 μm. II. A. thaliana primary roots growth after the direct exposure of both shielded and non-shielded seedlings to UV-B (27,2 and 68 kJ/m2). III. Primary roots morphology of shielded A. thaliana seedlings in 24 h after exposure to UV-B (27.2 and 68 kJ/m2): А − control; B - 27.2 kJ/m2; C − 68 kJ/m2. Bar - 200 μm.
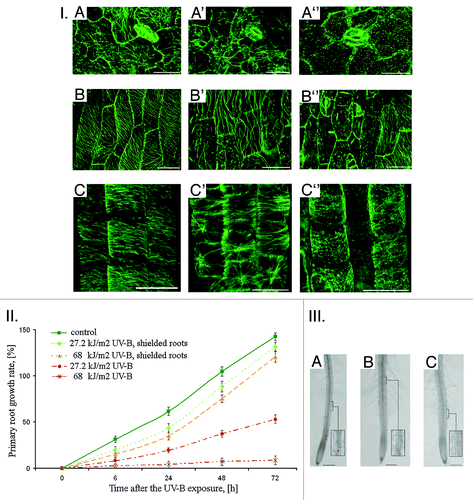
The most resistant were cortical МTs in stomatal cells of adaxial leaf surface (Fig. One (I), A-A”) organized in radial network of toughly adjacent bundles. In contrast, cortical MTs of adaxial epidermal leaf and hypocotyl cells became randomized (27.2 kJ/m2, , A’, B’) and partially depolymerized after the UV-B exposure (68 kJ/m2, , A,” B”) as compared with the radially and obliquely oriented MTs in cells of non-irradiated roots (Fig. One (I), A, B). In cells of non-irradiated abaxial side the organization of cortical MTs remained unaltered similar to control (Fig. One (I), А). Although earlier it was shown that the UV-B-induced inhibition of A. thaliana leaf plates growth after chronic UV-B exposure is not supported by МТs reorganization in adaxial leaf surface epidermal cells,Citation25,Citation27 we suppose that MTs could be a good candidate for UV-B-signal perception and its further transduction. However, their exact position in UV-B-related signaling cascades remains to be elucidated.
In the same time, in 2 h after the UV-B exposure cortical МTs in epidermal cells of primary root transition zone of shielded A. thaliana seedlings also became evidently randomized (Fig. One (I), C’) and depolymerized in dose-dependent manner (Fig. One (I), C”), while in the same cells of non-irradiated roots MTs oriented transversely (Fig. One (I), C). These observations are of special interest because the transition (distal elongation) zone is considered to be the main signaling-response nexus in the root as the inputs from hormonal and sensorial stimuli are integrated here and translated into signaling and motoric outputs.Citation28 Indeed, the transition zone cells are known to be sensitive to endogenous (auxin, ethylene, extracellular Ca2+) and exogenous factors (mechanical pressure, aluminum, pathogens).Citation29 In turn, cortical MTs in epidermal cells of the transition zone are exceptionally responsive to fluctuations of auxinsCitation30 and nitric oxide content,Citation4 protein kinase/phosphatase inhibitorsCitation31 and cold treatment as well.Citation32 We have shown also that immediately after the UV-B exposure (13.6–68 kJ/m2) cortical MTs in both transition and elongation zones depolymerized rapidly.Citation26
Furthermore, UV-B-induced MTs reorganization in root cells of shielded A. thalianа seedlings was accompanied by primary root growth inhibition (, (II),,) and epidermal cells swelling together with the intense root hairs formation in differentiation zone (, (III), B, C) as compared with non-shielded irradiated ones (, (II), ,; , (II), A, respectively) that points out to the activation of the morphogenetic processes.
As cytoskeleton reorganization was revealed mainly in vitro in protoplasts and other cell cultures,Citation21-Citation24 we have obtained the complementary results on cells of Nicotiana tabacum BY-2 suspension culture expressing gfp-mbd (microtubule-binding domain of MAP4) as a suitable cell model for in vivo MTs visualization.Citation33 Since BY-2 cells are more resistant to UV-B exposure as compared with A. thaliana seedlings, the higher doses of UV-B (34, 81 and 135 kJ/m2) were used33. In 3 h after the irradiation a dose-dependent depolymerization of both interphase (Fig. Two (I,II), А”-C”) and mitotic (not shown) MTs clearly corresponded to cytoplasm shrinkage (, А-C), chromatin condensation (, А'–C'), cytoplasm vacuolization (, II, А-В) and micronuclei formation (, II, А'-В').
Figure 2. Alterations of general ВУ-2 cells morphology, nuclei shape and interphase MTs organization in 3 h after the UV-B exposure (A - 34 kJ/m2; B - 81 kJ/m2;C -135 kJ/m2): I. A-C - cytoplasm shrinkage; A'-C'- chromatin condensation, DAPI (4',6-diamidino-2-phenylindole) nuclei staining; A”-C” - МТs randomization/depolymerization. II. A-C - cytoplasm vacuolization; A'-C'- micronuclei formation, DAPI staining; A”-C” - МТs randomization/depolymerization. Bar - 50 μm.
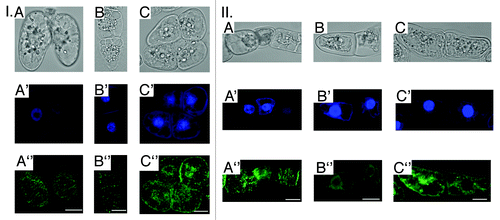
Hence, the key hallmarks of the programmed cell death (PCD) were found after UV-B exposure in BY-2 cells. Here we clearly demonstrate the involvement of MTs in UV-B response and an apoptosis development under UV-B stress in different plant cells. However, the involvement of MTs in PCD progression in plant cells is still scarce and need to be further investigated.
Conclusions
In summary, these data represent, to our knowledge, the first steps in understanding of the MTs involvement into signaling events under the UV-B response in plant cells. However, the detailed mechanisms of cytoskeleton reorganization during the UV-B response are complex and varied, and much still remain to be elucidated, especially in terms of the signaling molecules and related transduction pathways.
| Abbreviations: | ||
| UV-B | = | ultraviolet irradiation with wavelengths in the range of 280−315 nm |
| MT | = | microtubules |
| PCD | = | programmed cell death |
Disclosure of Potential Conflicts of Interest
There were no potential conflicts of interest to disclose.
Acknowledgments
This work was partially funded by the grant F47/072 of the State Fund of Fundamental Researches (Ukraine) for Dr. A. Yemets.
References
- Ehrhardt DW, Shaw SL. Microtubule dynamics and organization in the plant cortical array. Annu Rev Plant Biol 2006; 57:859 - 75; http://dx.doi.org/10.1146/annurev.arplant.57.032905.105329; PMID: 16669785
- Staiger CJ. Signaling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol 2000; 51:257 - 88; http://dx.doi.org/10.1146/annurev.arplant.51.1.257; PMID: 15012193
- Foster R, Mattsson O, Mundy J. Plants flex their skeletons. Trends Plant Sci 2003; 8:202 - 4; http://dx.doi.org/10.1016/S1360-1385(03)00061-X; PMID: 12758035
- Yemets AI, Krasylenko YA, Lytvyn DI, Sheremet YA, Blume YB. Nitric oxide signalling via cytoskeleton in plants. Plant Sci 2011; 181:545 - 54; http://dx.doi.org/10.1016/j.plantsci.2011.04.017; PMID: 21893251
- Blume YB, Krasylenko YA, Yemets AI. Effects of phytohormones on the cytoskeleton of plant cell. Russ J Plant Physiol 2012; 59:515 - 29; http://dx.doi.org/10.1134/S1021443712040036
- Lanza M, Garcia-Ponce B, Castrillo G, Catarecha P, Sauer M, Rodriguez-Serrano M, et al. Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Dev Cell 2012; 22:1275 - 85; http://dx.doi.org/10.1016/j.devcel.2012.04.008; PMID: 22698285
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 2006; 45:113 - 22; http://dx.doi.org/10.1111/j.1365-313X.2005.02615.x; PMID: 16367958
- Hwang JU, Lee Y. Abscisic acid-induced actin reorganization in guard cells of dayflower is mediated by cytosolic calcium levels and by protein kinase and protein phosphatase activities. Plant Physiol 2001; 125:2120 - 8; http://dx.doi.org/10.1104/pp.125.4.2120; PMID: 11299391
- Liu X, Zhang SQ, Lou CH. Involvement of Ca2+ in stomatal movement of Vicia faba L. regulated by nitric oxide. J Plant Physiol Mol Biol 2003; 29:342 - 6
- Yemets AI, Lloyd C, Blume YB. Plant tubulin phosphorylation and its role in cell cycle progression. 2008; The Plant Cytoskeleton: Key Tool for Agro-Biotechnology (Eds. Blume YaB, Baird WV, Yemets AI, Breviario D). Dordrecht: Springer, p. 145-59.
- Yemets A, Sheremet Y, Vissenberg K, Van Orden J, Verbelen J-P, Blume YB. Effects of tyrosine kinase and phosphatase inhibitors on microtubules in Arabidopsis root cells. Cell Biol Int 2008; 32:630 - 7; http://dx.doi.org/10.1016/j.cellbi.2008.01.013; PMID: 18343165
- Blume Y, Yemets A, Sheremet Y, Nyporko A, Sulimenko V, Sulimenko T, et al. Exposure of beta-tubulin regions defined by antibodies on an Arabidopsis thaliana microtubule protofilament model and in the cells. BMC Plant Biol 2010; 10:29; http://dx.doi.org/10.1186/1471-2229-10-29; PMID: 20167106
- Takemoto D, Hardham AR. The cytoskeleton as a regulator and target of biotic interactions in plants. Plant Physiol 2004; 136:3864 - 76; http://dx.doi.org/10.1104/pp.104.052159; PMID: 15591444
- Higaki T, Sano T, Hasezawa S. Actin microfilament dynamics and actin side-binding proteins in plants. Curr Opin Plant Biol 2007; 10:549 - 56; http://dx.doi.org/10.1016/j.pbi.2007.08.012; PMID: 17936064
- Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol 2008; 20:71 - 6; http://dx.doi.org/10.1016/j.ceb.2007.11.010; PMID: 18226514
- Nick P. 2008. Microtubules as Sensors for Abiotic Stimuli. In Nick P, ed, Plant Microtubules. Springer-Verlag, Berlin, pp 175-203.
- McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, Madronich S. Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci 2011; 10:182 - 98; http://dx.doi.org/10.1039/c0pp90034f; PMID: 21253660
- Jansen MAK. Ultraviolet-B radiation effects on plants: induction of morphogenic responses. Physiol Plant 2002; 116:423 - 9; http://dx.doi.org/10.1034/j.1399-3054.2002.1160319.x
- Hollósy F. Effects of ultraviolet radiation on plant cells. Micron 2002; 33:179 - 97; http://dx.doi.org/10.1016/S0968-4328(01)00011-7; PMID: 11567887
- Rozema J, van de Staaij J, Björn LO, Caldwell MM. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol 1997; 12:22 - 8; http://dx.doi.org/10.1016/S0169-5347(96)10062-8; PMID: 21237957
- Staxèn I, Bergounioux C, Bornman JF. Effect of ultraviolet radiation on cell division and microtubules organization in Petunia hybrida protoplasts. Protoplasma 1993; 173:70 - 6; http://dx.doi.org/10.1007/BF01378863
- Zhang MP, Han R, Shan YJ, Li Y, Wu L, Tian WH, et al. Effects of enhanced UV-B radiation on the cell mitosis of the callus in wheat. 2009; 3rd Int. Conference on Bioinformat. Biomed. Engineer. 1-4. (Abstr.).
- Guo AH, Gao LM, Li YF, Han R. Influence on microtubule in wheat mesophyll cell exposed to enhanced ultraviolet-B radiation and He-Ne laser irradiation. CNKI J Guihaia. 2: 2010; DOI: CNKI:SUN:GXZW.0.2010-02-021.
- Chen HZ, Zhai JR, Du MT, Han R. Influence of enhanced UV-B radiation on F-actin in wheat division cells. Plant Divers Res 2011; 33:306 - 10
- Jacques E, Hectors K, Guisez Y, Prinsen E, Jansen MAK, Verbelen JP, et al. UV radiation reduces epidermal cell expansion in Arabidopsis thaliana leaves without altering cellular microtubule organization. Plant Signal Behav 2011; 6:1 - 3; http://dx.doi.org/10.4161/psb.6.1.14127; PMID: 21307662
- Krasylenko YA, Yemets AI, Sheremet YA, Blume YB. Nitric oxide as a critical factor for perception of UV-B irradiation by microtubules in Arabidopsis.. Physiol Plant 2012; 145:505 - 15; http://dx.doi.org/10.1111/j.1399-3054.2011.01530.x; PMID: 21973209
- Hectors K, Jacques E, Prinsen E, Guisez Y, Verbelen JP, Jansen MAK, et al. UV radiation reduces epidermal cell expansion in leaves of Arabidopsis thaliana.. J Exp Bot 2010; 61:4339 - 49; http://dx.doi.org/10.1093/jxb/erq235; PMID: 20702567
- Baluška F, Mancuso S, Volkmann D, Barlow PW. Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 2010; 15:402 - 8; http://dx.doi.org/10.1016/j.tplants.2010.04.007; PMID: 20621671
- Baluška F, Volkmann D, Barlow PW. A polarity crossroad in the transition growth zone of maize root apices: cytoskeletal and developmental implications. J Plant Growth Regul 2001; 20:170 - 81; http://dx.doi.org/10.1007/s003440010013
- Takahashi H, Kawahara A, Inoue Y. Ethylene promotes the induction by auxin of the cortical microtubule randomization required for low-pH-induced root hair initiation in lettuce (Lactuca sativa L.) seedlings. Plant Cell Physiol 2003; 44:932 - 40; http://dx.doi.org/10.1093/pcp/pcg119; PMID: 14519775
- Sheremet YA, Yemets AI, Vissenberg K, Verbelen J-P, Blume YB. Effects of inhibitors of serine/threonine protein kinases on Arabidopsis thaliana root morphology and microtubule organization in its cells. Cell Tissue Biol 2010; 4:389 - 98; http://dx.doi.org/10.1134/S1990519X10040139
- Sheremet YA, Yemets AI, Blume YB. Inhibitors of tyrosine kinases and phosphatases as a tool for the investigation of microtubule role in plant cold response. Cytol Genet 2012; 46:1 - 8; http://dx.doi.org/10.3103/S0095452712010112
- Lytvyn DI, Yemets AI, Blume YB. UV-B overexposure induces programmed cell death in a BY-2 tobacco cell line. Environ Exp Bot 2010; 68:51 - 7; http://dx.doi.org/10.1016/j.envexpbot.2009.11.004