Abstract
Aphids infest many plants and cause damage by depriving them of nutrients and by transmitting many viral diseases. Aphid infestation and arbovirus transmission are controlled by establishment (or not) of a compatible reaction between the insects and the plants. This reaction is the result of defense reactions of the plant and counter-defense reactions of the parasite. Contrarily to plant-bacteria, plant-fungi and plant-herbivorous insects pathosystems, the plant-aphid pathosystem is understudied, although recent advances have begun to uncover some of its details. Especially the very early steps in plant-aphid interactions are hardly known. We here resume the present knowledge of these interactions. We discuss further how an aphid-transmitted plant virus that is transmitted during the first moments of the plant-aphid encounter, might help to study the very early plant aphid interactions.
Aphids are with more than 4,000 species the largest group of insect phloem feeders and they are distributed worldwide.Citation1 They damage many crops by removing nutrients from plants and by transmitting plant pathogens, especially viruses. Aphids are perfect and efficient vectors, because they acquire and deliver pathogens directly from and into living cells. This is due to their particular feeding behavior; upon alighting on a new plant, aphids test them for suitability by probing punctures into leaves. During these punctures, aphids insert their stylets into the tissue, where they meander in between epidermal and mesophyll cells. The stylets are not in direct contact with the cell wall. Rather, a gelling saliva is secreted constantly from the stylet tips and forms a sheath around the stylets, insulating them from the tissue. Occasionally the stylets enter into cells. If this happens, aphids secret some watery saliva into the cell before ingesting a little bit of its contents. This allows testing and accepting or refusing the host within a few minutes. If the host is accepted, the stylets will penetrate deeper into the tissue until they tap the sieve tubes. There the stylets can remain for hours taking up the sieve tube sap, whose amino acids constitute the principal nutrient source for aphids. (reviewed in ref. Citation2).
Reports on saliva composition are somewhat confounding. The composition of gelling or sheath saliva is assumed to be quite similar among different aphid species and gelling is assumed to be caused by oxidation of sulphydryl groups, whereas watery saliva composition seems to be more variable.Citation3,Citation4 Watery saliva secreted during phloem feeding contains compounds interacting with sieve tube components, for example to prevent sieve tube clogging.Citation5 It is also speculated that watery saliva secreted into the phloem is different from saliva secreted into parenchym cells and that the method used to collect saliva might also influence saliva composition.Citation2 Consequently, different saliva proteomes and enzyme activities have been published.Citation6-Citation8 Recent work combines proteomic and genetic approaches to establish the aphid saliva secretome.Citation9,Citation10
From the great diversity of aphid species, only a few are able to grow on a given plant species. In fact, most aphid species are oligophagous. The reason therefore seems to be, besides metabolic incompatibility, specific recognition of the aphid attack by the plant, followed by initiation of defense reactions. Likewise, aphids discriminate between host and non-host plants, an item we do not consider here. These interactions require an initial recognition event that—seen from the plant's side of view—can origin from the mechanical stress exercised by the aphid stylet movement or from a chemical stress elicited by component(s) of the sheath or watery saliva. This very early recognition event will be transduced by one or several signaling pathways and eventually be translated into the various plant responses: from fast posttranslational modifications such as phosphorylation to transcriptional changes and metabolic reprogramming leading for example to the production of toxic compounds for the pathogen on the medium to long range.Citation11,Citation12 In this review, we resume the early events of plant responses to aphid attack and we discuss about a case where an aphid-transmitted plant virus responds immediately to the presence of the insect to organize its transmission.
The very early interactions between aphids and plants are hardly known. Stylet penetration into tissues and salivation are the earliest events in direct plant-aphid interactions and presents a potential recognition source. For example, the mechanical stress provoked by the stylets gliding in the tissue might elicit a plant reaction. In this regard it has been reported that touching cells with a microelectrode tip induces various subcellular changes. For instance, reactive oxygen species (ROS) and calcium influx are induced during touch response.Citation13 Similarly, organelles like nuclei, Golgi bodies, peroxisomes and the cytoskeleton react to mechanical stimulation within minutes by reorientation.Citation14,Citation15 It is not known whether aphids also trigger such changes, even though they exercise certainly mechanical stress during probing. However, plant defenses in response against chewing herbivorous insects (for example caterpillars) are not induced primarily by the mechanical clipping and chewing stress but rather by saliva and foregut secretions that are released into the wounded tissue.Citation16 Analogously, aphid feeding might also be recognized—besides by mechanical stress itself—by a chemical elicitor (originating either from the damaged plant tissue itself or by the aphid) and plant-aphid interactions might be modulated by effectors. Very little is known about the nature of these molecules. Recent advances in identification of aphid saliva proteins and their analysis have put into evidence a role of some of these proteins in both triggering (elicitors) and counteracting (effectors) plant defenses. Interestingly, some of these molecules act species-specific, for example COO2, MP1, MP2 and MP10, whereas others have a much broader specificity, for example glucose oxidase where the protein itself or its enzymatic reaction products such as H2O2 are assumed to inhibit and/or elicit plant defense responses.Citation9,Citation17,Citation18 Consequently, aphid elicitors and effectors might derive from a highly conserved protein (comparable with the bacterial flagellin or fungal chitin/chitosan) shared by many aphids or they might be specific to an aphid/plant couple, as is the case especially for effectors.Citation18
These findings are in line with the zigzag model that aims to explain the plant immune system.Citation19 In a first step, pathogen elicitors—(either derived directly from the pathogen or plant degradation products generated during pathogen activity) are recognized with rather broad specificity as so-called pathogen-associated molecular patterns (PAMPs) by corresponding plant pattern recognition receptors (PRRs). This results in PAMP-triggered immunity (PTI) that inhibits infestation. PTI can be neutralized by pathogen effectors that interfere with PTI and re-establish plant susceptibility. In the next step, plant NB-LRR proteins recognize specifically these pathogen effectors and induce effector-triggered immunity (ETI) that results in defense responses such as the hypersensitive reaction. In turn, pathogens can fight NB-LRR proteins or proteins in the ETI pathway by ETI suppressors, the plant can counteract ETI suppressors and so on. In the case of herbivore-plant interactions, a similar model has been proposed where the term PAMP is replaced by HAMP (herbivore-AMP). Aphid HAMP could be glucose oxidase, pectinase and other enzymes or their reaction products,Citation20 and not yet fully characterized saliva low molecular weight proteins/peptides.Citation21 Aphid effectors are saliva proteins such as MP10, MP42 and COO2 (reviewed in ref. Citation22). Finally, plant NB-LRR proteins involved in aphid ETI are the Vat and Mi-1 gene products, classical R genes (reviewed in ref. Citation23).
Functional analyses of some of these proteins showed that for example MP10 induced chlorosis and weakly induced cell death in Nicotiana benthamiana, and suppressed the oxidative burst induced by the bacterial PAMP flagellin 22 peptide (flg22),Citation9,Citation24 establishing a role as an effector for this protein. The functioning of other aphid effectors remains unclear. Besides insect proteins, also chitin, plant cell wall and plant cell degradation products might play a role in the recognition events.
What are the very early events that are possibly triggered by these or yet unidentified elicitors? The first known reactions are electrical events: membrane depolarization and ion fluxes that can be propagated often through long distances as voltage-induced and ion channel-mediated action potentials, system potentials that are induced by plasma membrane depolarization and for example be mediated by proton pump activation, and variation potentials that are induced by rapid turgor increase and driven by proton pump inhibition.Citation25-Citation27 Calcium fluxes seem to play a very important role in these initial events; and aphid effector molecules might interact with calcium channels or other components of the calcium signaling cascade, either intra- or extracellularly. Will and coworkers observed that aphid saliva components chelated calcium and prevented forisome-mediated phloem clogging.Citation5 Although this observation is probably not relevant for initial plant-aphid recognition, it does illustrate nicely that aphid components may interact with calcium and influence calcium signaling.
Another signaling pathway that seems to be involved in the very early plant-aphid interactions, are ROS. These products have a dual role: at low concentrations they act as second messengers involved in cell signaling and at high concentration they play a role in the direct defense. Indeed, ROS are toxic to insects; they induce a hypersensitive response and trigger the plant defense pathway locally and in other tissues.Citation28 Whereas hypersensitive responses after aphid infestation have been reported (for example ref. Citation29), virtually nothing is known about ROS signaling and early plant-aphid interactions. Work on Fall armyworm larvae foraging on lima bean showed that mechanical wounding and herbivore activity induced ROS and calcium signaling.Citation25 However, the ROS and calcium response were weaker during herbivore activity, again indicating that insect saliva might manipulate normal plant defense responses. Besides local responses, also rapid long distance signaling of ROS has been reported.Citation30 Taken together, the domain of early plant-aphid interactions is still largely unexplored.
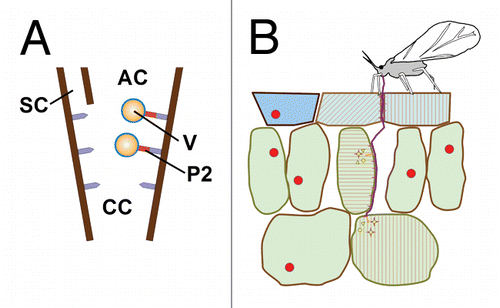
Many plant viruses are spread by aphid vectors. This means that there are interactions between the aphids, the plants and the viruses during virus acquisition and inoculation. It is likely that viruses might exploit plant-aphid interactions for transmission and reveal useful for studying these interactions (reviewed in ref. Citation31). Of special interest for studying early interactions between plants and aphids could be viruses using the non-circulative transmission strategy, because these viruses are acquired and released by the aphids exactly during the very early plant-aphid interactions. So they might be witnesses of the early plant-aphid interactions (and virus acquisition/inoculation processes could even be controlled by them). Recent work by us suggests that this is indeed the case for the transmission of cauliflower mosaic virus (CaMV).Citation32 This virus forms specialized viral transmission bodies (TB) in the cytoplasm of infected plant cells that are required for transmission (reviewed in refs. Citation31 and Citation33). The TB contains among others the viral protein P2 that links the CaMV particles to a receptor localized in the aphid stylets (). We have accumulated evidence that the TB reacts instantly (within seconds) to the presence of the vector, i.e., to the stylet punctures and/or aphid saliva components: in normal, unstressed tissues, the TB is a well-defined spherical inclusion body. However, upon aphid stress there is first a massive influx of tubulin into TB and then its key component P2 redistributes ultra-rapidly onto microtubules throughout the cell periphery.Citation32 At the same time and with the same rapidity, virus particles are recruited from the cytoplasmic virus factories and also bind to microtubules (Bak et al., unpublished). P2 and virus particles united on the microtubules are then acquired easily by the aphid (). Remarkably, P2 dissociates from the microtubules and accumulates into a new TB after aphid removal and also the virus particles return to the virus factories and are then ready for another round of transmission. We found that the TB reaction is not induced by every stress: of all the stresses tested, only wounding or the chemicals CO2 and NaN3 induced typical TB dissociation, whereas heat shock and mechanical stress induced mere tubulin accumulation in TB, and all other stresses had no effect at all. This seems to indicate that mechanical stress like stylet gliding in the tissue only triggers partially the TB response and that a chemical signal (either a saliva component and/or a plant wounding stress marker) is required for the complete TB reaction. Our work showed also that the complete TB reaction (i.e., formation of P2 networks on microtubules) was restricted to the cells in contact with salivary sheaths, and this apparently independent of the fact whether or not they had been pierced by the stylets. This again indicates that a sheath saliva component is the elicitor and that an effector protein might be required for complete TB transformation. Unlike for chewing herbivore stress or prolonged aphid stress (48 h),Citation21 no immediate propagation of the stress to other regions of the same tissue or distant parts of the plants occurred. This suggests that the initial early signals triggered by aphids and chewing/clipping insects are translated into different responses.
Figure 1. CaMV exploits early plant-aphid interactions for transmission. (A) The CaMV transmissible complex is composed of the virus particle (V) and the viral helper protein P2 (red) that binds to a receptor (mauve) in the common canal (CC) in the stylet tips. AC, alimentary canal; SC, salivary canal. (B) An aphid inserts its stylets into the tissue where they meander in between the cells. During the stylet movement sheath saliva is constantly secreted that polymerizes to form a sheath (magenta). Some cells are punctured by the stylets. In this case, the aphid secrets some watery saliva (yellow) into the cell and then aspires some cytoplasm. The stylets will penetrate further into the tissue until they are inserted into the sieve tubes (not shown), or if the interaction is incompatible, the stylets will be retracted and the aphid either tries to puncture elsewhere on the same plant or it takes off for another plant. Aphid elicitors and/or effectors can be sheath or watery saliva components that are recognized by plant pattern recognition receptors and result in a recognition reaction (light magenta). CaMV forms in infected plant cells transmission bodies (TB, red circles) that contain basically the viral helper protein P2. The mechanical and/or chemical stress provoked by the aphid stylet activity triggers an early plant response (membrane depolarization, calcium and other ion fluxes, elicitor-plant pattern recognition receptor interactions). This early response is “sensed” by the TB, which disintegrates instantly and redistributes its P2 contents onto microtubules (red hatches in touched and penetrated cells). This P2 morph is acquirable by the aphid and allows for efficient transmission. After departure of the vector, the TBs reform (not shown). The TB reaction is detected easily by immunofluorescence microscopy and might be used to screen for aphid elicitors and effectors.
An interesting feature of the TB response is the rapidity of both its onset and of its reversion. Because the TB reaction is so fast, we postulate that the TB is responsive to a stimulus created during the very early plant-aphid reaction. But whereas the plant will eventually and upon persistence of infestation transduce this signal in the middle or long-term into a defense reaction, the TB will use this stimulus only for the TB reaction and will have reverted to back to ground state long before the plant reactions establish. Thus the TB response to aphid attack diverts from the plant response after the initial recognition event and is apparently independent from it.
The TB reaction is also triggered in isolated infected protoplasts.Citation32 This protoplast assay can thus be used in a medium throughput system to phenotype CaMV transmission and eventually to study transmission of other viruses.Citation34 Indeed, it is possible that other plant or animal arboviruses also interfere with pathways for perception of aphids or corresponding vectors (white flies, mosquitoes, ticks) to know when it is time for transmission and prepare to this event. This is a very important question for future research in virology. Alternatively, this system might be useful as a sensor to identify aphid elicitors and effectors on the cellular level; CaMV-infected plant cells are incubated with various molecules and positive reacting molecules are revealed by the TB phenotype and/or measurement of ROS and calcium levels, etc. This will allow for a first time a screen specifically for early acting elicitors and effectors during plant-aphid interactions.
Acknowledgments
Our work is funded by ANR (Agence National de la Recherche, France) grants BLAN07-2-192768 awarded to S.B. and ANR12-BSV7-005-01 awarded to M.D. M.D. and S.B. also appreciate highly project funding by the SPE department of INRA.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Blackman R, Eastop V. Aphids on the World’s Trees: An Identification and Information Guide. Wallingford, UK: CABI. 1st Ed. 1994.
- Tjallingii WF. Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 2006; 57:739 - 45; http://dx.doi.org/10.1093/jxb/erj088; PMID: 16467410
- Will T, Steckbauer K, Hardt M, van Bel AJE. Aphid gel saliva: sheath structure, protein composition and secretory dependence on stylet-tip milieu. PLoS ONE 2012; 7:e46903; http://dx.doi.org/10.1371/journal.pone.0046903; PMID: 23056521
- Will T, Kornemann SR, Furch ACU, Tjallingii WF, van Bel AJE. Aphid watery saliva counteracts sieve-tube occlusion: a universal phenomenon?. J Exp Biol 2009; 212:3305 - 12; http://dx.doi.org/10.1242/jeb.028514; PMID: 19801435
- Will T, Tjallingii WF, Thönnessen A, van Bel AJE. Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA 2007; 104:10536 - 41; http://dx.doi.org/10.1073/pnas.0703535104; PMID: 17553961
- Miles PW. Aphid Saliva. Biol Rev Camb Philos Soc 1999; 74:41 - 85; http://dx.doi.org/10.1017/S0006323198005271
- Carolan JC, Fitzroy CIJ, Ashton PD, Douglas AE, Wilkinson TL. The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics 2009; 9:2457 - 67; http://dx.doi.org/10.1002/pmic.200800692; PMID: 19402045
- Harmel N, Létocart E, Cherqui A, Giordanengo P, Mazzucchelli G, Guillonneau F, et al. Identification of aphid salivary proteins: a proteomic investigation of Myzus persicae. Insect Mol Biol 2008; 17:165 - 74; http://dx.doi.org/10.1111/j.1365-2583.2008.00790.x; PMID: 18353105
- Bos JIB, Prince D, Pitino M, Maffei ME, Win J, Hogenhout SA. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet 2010; 6:e1001216; http://dx.doi.org/10.1371/journal.pgen.1001216; PMID: 21124944
- Carolan JC, Caragea D, Reardon KT, Mutti NS, Dittmer N, Pappan K, et al. Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): a dual transcriptomic/proteomic approach. J Proteome Res 2011; 10:1505 - 18; http://dx.doi.org/10.1021/pr100881q; PMID: 21226539
- Maffei ME, Mithöfer A, Boland W. Before gene expression: early events in plant-insect interaction. Trends Plant Sci 2007; 12:310 - 6; http://dx.doi.org/10.1016/j.tplants.2007.06.001; PMID: 17596996
- Giordanengo P, Brunissen L, Rusterucci C, Vincent C, van Bel A, Dinant S, et al. Compatible plant-aphid interactions: how aphids manipulate plant responses. CR Biol 2010; 333:516 - 23; http://dx.doi.org/10.1016/j.crvi.2010.03.007; PMID: 20541163
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 2009; 21:2341 - 56; http://dx.doi.org/10.1105/tpc.109.068395; PMID: 19654264
- Hardham AR, Takemoto D, White RG. Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biol 2008; 8:63; http://dx.doi.org/10.1186/1471-2229-8-63; PMID: 18513448
- Qu L-H, Sun M-X. The plant cell nucleus is constantly alert and highly sensitive to repetitive local mechanical stimulations. Plant Cell Rep 2007; 26:1187 - 93; http://dx.doi.org/10.1007/s00299-007-0343-6; PMID: 17396239
- Bricchi I, Leitner M, Foti M, Mithöfer A, Boland W, Maffei ME. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: early signaling and volatile emission in lima bean (Phaseolus lunatus L.). Planta 2010; 232:719 - 29; http://dx.doi.org/10.1007/s00425-010-1203-0; PMID: 20563731
- Tian D, Peiffer M, Shoemaker E, Tooker J, Haubruge E, Francis F, et al. Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS ONE 2012; 7:e36168; http://dx.doi.org/10.1371/journal.pone.0036168; PMID: 22558369
- Pitino M, Hogenhout SA. Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol Plant Microbe Interact 2013; 26:130 - 9; http://dx.doi.org/10.1094/MPMI-07-12-0172-FI; PMID: 23035913
- Jones JDG, Dangl JL. The plant immune system. Nature 2006; 444:323 - 9; http://dx.doi.org/10.1038/nature05286; PMID: 17108957
- Liu Y, Wang W-L, Guo G-X, Ji X-L. Volatile emission in wheat and parasitism by Aphidius avenae after exogenous application of salivary enzymes of Sitobion avenae. Entomol Exp Appl 2009; 130:215 - 21; http://dx.doi.org/10.1111/j.1570-7458.2008.00822.x
- De Vos M, Jander G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ 2009; 32:1548 - 60; http://dx.doi.org/10.1111/j.1365-3040.2009.02019.x; PMID: 19558622
- Hogenhout SA, Bos JIB. Effector proteins that modulate plant-insect interactions. Curr Opin Plant Biol 2011; 14:422 - 8; http://dx.doi.org/10.1016/j.pbi.2011.05.003; PMID: 21684190
- Dogimont C, Bendahmane A, Chovelon V, Boissot N. Host plant resistance to aphids in cultivated crops: genetic and molecular bases, and interactions with aphid populations. CR Biol 2010; 333:566 - 73; http://dx.doi.org/10.1016/j.crvi.2010.04.003; PMID: 20541167
- Arimura G-I, Ozawa R, Maffei ME. Recent advances in plant early signaling in response to herbivory. Int J Mol Sci 2011; 12:3723 - 39; http://dx.doi.org/10.3390/ijms12063723; PMID: 21747702
- Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 2004; 134:1752 - 62; http://dx.doi.org/10.1104/pp.103.034165; PMID: 15051862
- Zimmermann MR, Maischak H, Mithöfer A, Boland W, Felle HH. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol 2009; 149:1593 - 600; http://dx.doi.org/10.1104/pp.108.133884; PMID: 19129416
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 2010; 44:1 - 24; http://dx.doi.org/10.1146/annurev-genet-102209-163500; PMID: 20649414
- Kuśnierczyk A, Winge P, Jørstad TS, Troczyńska J, Rossiter JT, Bones AM. Towards global understanding of plant defence against aphids—timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ 2008; 31:1097 - 115; http://dx.doi.org/10.1111/j.1365-3040.2008.01823.x; PMID: 18433442
- Moloi MJ, van der Westhuizen AJ. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J Plant Physiol 2006; 163:1118 - 25; http://dx.doi.org/10.1016/j.jplph.2005.07.014; PMID: 17032617
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2009; 2:ra45; http://dx.doi.org/10.1126/scisignal.2000448; PMID: 19690331
- Blanc S, Uzest M, Drucker M. New research horizons in vector-transmission of plant viruses. Curr Opin Microbiol 2011; 14:483 - 91; http://dx.doi.org/10.1016/j.mib.2011.07.008; PMID: 21788152
- Martinière A, Bak A, Macia J-L, Lautredou N, Gargani D, Doumayrou J, et al. A virus responds instantly to the presence of the vector on the host and forms transmission morphs. eLife 2013; 2:e00183; http://dx.doi.org/10.7554/eLife.00183; PMID: 23358702
- Bak A, Irons SL, Martinière A, Blanc S, Drucker M. Host cell processes to accomplish mechanical and non-circulative virus transmission. Protoplasma 2012; 249:529 - 39; http://dx.doi.org/10.1007/s00709-011-0328-8; PMID: 21984344
- Martinière A, Macia J-L, Bagnolini G, Jridi C, Bak A, Blanc S, et al. VAPA, an innovative “virus-acquisition phenotyping assay” opens new horizons in research into the vector-transmission of plant viruses. PLoS ONE 2011; 6:e23241; http://dx.doi.org/10.1371/journal.pone.0023241; PMID: 21853093