Abstract
A direct somatic embryogenesis protocol was developed for four cultivars of Nicotiana species, by using leaf disc as an explant. Direct somatic embryogenesis of Nicotiana by using BAP and IAA has not been investigated so far. This method does not require formation of callus tissues which leads to somaclonal variations. The frequency of somatic embryogenesis was strongly influenced by the plant growth hormones. The somatic embryos developing directly from explant tissue were noticed after 6 d of culture. Somatic embryogenesis of a high frequency (87–96%) was observed in cultures of the all four genotypes (Nicotiana tabacum, N. benthamiyana, N. xanthi, N. t cv petihavana). The results showed that the best medium for direct somatic embryogenesis was MS supplemented with 2.5 mg/l, 0.2 mg/l IAA and 2% sucrose. Subculture of somatic embryos onto hormone free MS medium resulted in their conversion into plants for all genotypes. About 95% of the regenerated somatic embryos germinated into complete plantlets. The plants showed morphological and growth characteristics similar to those of seed-derived plants. Explants were transformed using Agrobacterium tumifacious LBA4404 plasmid pCAMBIA1301 harboring the GUS gene. The regenerated transgenic plants were confirmed by PCR analysis and histochemical GUS assay. The transformation efficiency obtained by using the Agrobacterium- mediated transformation was more than 95%. This method takes 6 wk to accomplish complete transgenic plants through direct somatic embryogenesis. The transgenic plantlets were acclimatized successfully with 98% survival in greenhouse and they showed normal morphological characteristics and were fertile. The regeneration and transformation method described herein is very simple, highly efficient and fast for the introduction of any foreign gene directly in tobacco through direct somatic embryogenesis.
Introduction
Tobacco is a model plant which belongs to solanaceae family and is being used also a bioreactor to introduce more genes to the plant to produce therapeutic proteins. For example, it is possible to produce up to 360 million doses of an anthrax vaccine in one acre of tobacco.Citation1 The generation of transgenic plants via Agrobacterium-mediated transformation or particle bombardment depends mainly on the standardization of an efficient tissue culture and regeneration protocol. The regeneration of plants in vitro via somatic embryogenesis (SE) has some distinct features such as single-cell origin, the consequent low frequency of chimeras and the production of a high number of regenerates.Citation2,Citation3
Normally in plants, embryo-like structures can be originating from cells other than gametes (i.e., somatic cells) by avoiding the normal fertilization process, hence the term somatic embryos.Citation4 As somatic embryos are formed without any fertilization event they are genetically identical to the parent tissue and are therefore clones. SE is generally two types one is indirect SE where the embryos form from callus phase. The other one is direct SE where the embryos are formed from organized tissue without an intervening callus phase.Citation5 SE is a very important tool in plant biotechnology and can be utilized in a number of ways.Citation6-Citation8 SE can be used in genetic transformation studies. It can be used for studying molecular, regulatory and morphogenetic events in plant embryogenesis. For the production of large scale plants from the embryogenic line is the most commercially appealing application.Citation9 As with zygotic embryos, somatic embryos dormancy can be induced, hence long-term storage is possible. During regeneration, root and shoot formation is simultaneous thus eliminating the need for a root induction phase as with conventional micropropagation. SE can be used in the synthetic seed technology. Synthetic seeds have multiple advantages, including easy handling and transportation, potential long-term storage, higher scale-up capacity, uniformity in production, potential for automation of the whole production process, seeding of clonal varieties, and may provide a means for maintenance of elite germplasm.Citation10
Here we attempted to regenerate tobacco by using direct SE pathway. The direct SE of tobacco was first reported by StolarzCitation11 (1991); later Gill and SaxenaCitation12 (1993) reported the SE in Nicotiana tabacum L. induction by thidiazuron (TDZ) of direct embryo differentiation from cultured leaf discs. However, there have been reports of problems with conversion of TDZ-induced shoots into complete plantlets, poor elongation of shoots and inadequate rooting.Citation13,Citation14 Moreover regenerants originating from TDZ-induced adventitious shoots in woody plants often tend to be dwarf with shortened internodes.Citation14 Most of the protocols described in these studies have not been successfully used for the gene transformation.
Therefore, the aim of this research was to define the best conditions for direct somatic embryos induction and maturation from leaf discs of Nicotiana (Nicotiana tabacum, N. benthamiyana, N.xanthi, N.t cv petihavana) by studying the influence of culture media composition, light condition, culture system, and optimizing several key factors to utilize tobacco somatic embryos for Agrobacterium-mediated genetic transformation.
Results and Discussion
In the present investigation we have been successful in developing an efficient and highly reproducible protocol for direct SE along with improved Agrobacterium-mediated transformation from the leaf discs of four genotypes of Nicotiana (N. tabacum, N. benthamiyana, N. xanthi, N.t cv petihavana). Regeneration of plant via SE provides an excellent opportunity to generate a large number of plantlets in relatively short time duration. This method, although has been reported in many plant species, has been scantily documented for tobacco. In this study, we have optimized an efficient and a highly reproducible protocol for direct SE from the leaf discs of N. tabacum, N. benthamiyana, N. xanthi and N.t cv petihavana along with the stable transformation.
To generate plants/plantlets, leaf let is considered among the most reliable sources.Citation11 The explants of all genotypes were subcultured in MS medium (2% sucrose) with various combinations of BAP and IAA. The edges of leaf explants became bulged after three days of subculture. The bulged leaf portion later turned into pro embryos. However, most of the somatic embryos were obtained after two weeks of incubation under culture conditions. The highlight of the study is that we have by-passed the intermediate callus phase in generating plants via SE. These somatic embryos matured asynchronously through globular, heart, torpedo and cotyledonary shaped stages (). The development of the heart-shaped embryo was distinguished by the formation of a notch at the periphery (). All these developmental stages were observed over the entire surface of the leaf. The percentage of embryogenesis was significantly influenced by the concentration of the plant growth hormones used in the medium. In the present study, BAP and IAA were used for embryo induction and development, although somatic embryos emerged on all four media tested. Of all the concentrations tested, per explant maximum number of somatic embryos were generated in the MS medium supplemented with 2.5 mg/l BA + 0.2 mg/l IAA (). No morphological abnormal somatic embryos were detected. The ranges for somatic embryos per explants were varying from genotype to genotype. In case of N. tobaccum when BAP alone was used in the media at different concentration (1–5 mg/l) it turned to green callus with no further growth (data not shown). By combining the lower concentrations of both the hormones, BAP (1.5 mg/l) and IAA (0.1 mg/l), gave a maximum of 30 and a minimum of 20 embryos per explants (). Increasing the concentrations of both the hormones combinations (BAP and IAA) proved to increase the yield of somatic embryos per explant. The maximum number of somatic embryos per explant was 80 and minimum was 65 obtained by using 2.5 mg/l BAP and 0.2 mg/l IAA in N. tabacum. It was further found that, by using the above concentration (2.5 mg/l BAP and 0.2 mg/l IAA) resulted maximum somatic embryos (70, 70 and 75) in N. benthamiana, N. xanthi and N.t Petithavana, respectively. Further increased in the concentrations of BAP-3.0 and IAA-0.3 mg/l, was resulted decreased in the number of somatic embryos per explant. Which indicates the negative effect of BAP and IAA on multiplication of somatic embryo induction.
Figure 1. Various stages of direct somatic embryogenesis of Nicotiana tabacum. (A) Fused globular stage embryos;(B) early heart shape stage; (C) heart shaped stage; (D) early torpedo stage; (E) torpedo stage; (F) cotyledonary stage. Pictures were taken by Nikon I X -SMZ1500.
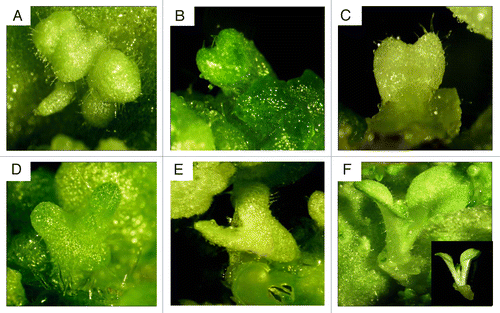
Table 1. Effects of different concentrations of plant growth hormones (IAA and BAP) used for direct somatic embryogenesis of Nicotiana genotypes in MS medium with 2% sucrose
The conversion of cotyledonary stage embryos to plantlets with well developed root systems (3–4 roots/culture) was attained in all embryos on hormones free MS medium within 7 d. The regenerated plantlets grew into well developed plants under greenhouse conditions. in vitro micropropagation of plants is achieved either by SE or organogenesis. In tobacco, cytokinin mediated induction of shoots via organogenesis is well known.Citation15-Citation17 Earlier, callus cultures maintained on kinetin and under high light intensity have exhibited different stages of SE in N. tabacum.Citation18 Later, StolarzCitation11 (1991) has reported generation of leaf disc cultured somatic embryos from a BAP and NAA supplemented medium. The addition of TDZ in culture media has also been documented to stimulate in vitro tobacco SE.Citation12 However, there were some problems of using TDZ in the culture media, which include the poor shoots elongation and insufficient rooting.Citation13,Citation14 Therefore, we have developed the direct SE without using the TDZ.
Agrobacterium-mediated transformation
Well matured leaf let were used to carry out in-vitro transformation studies. Sensitivity to antibiotic (hygromycin) selection is a factor that affects our ability to produce fertile transgenic tobacco. To optimize a suitable concentration of hygromycin to select the transformed tobacco somatic embryos, a kill curve experiment was performed on non-transformed explants cultured under conditions similar to the regeneration experiment, using media supplemented with different concentrations of hygromycin (10–50 mg l/l) and tested. Results exhibited a negative correlation between hygromycin concentrations and explant survival. The lethal dose of hygromycin, estimated at 30 mg/l, was chosen for selection of the transformed tissues (data not shown).
Explant was trimmed as earlier described and pre incubated for 2 d (). The effect of explants pre-culture on tobacco plant transformation is well documented.Citation19 Pre-incubation of cut leaf increases the transformation efficiency as compared with freshly cut leaf tissues. The improvement of transformation efficiency upon pre-incubation was reported in A. thaliana,Citation20,Citation21 tomato,Citation22 datura,Citation23 sugar beet,Citation24 watermelonCitation25 and P. nigra.Citation26 Exclusion of pre-culture condition, however, did not affect the transformation efficiency in kiwifruit and apple.Citation27 A. tumefaciens strain LBA 4404 harboring pCAMBIA1302 vector was used for transformation. An increase of OD > 1.0 resulted in tissue damage. In almond, a late log phase OD ~0.6 has been reported to be most effective to obtain high rates of transformation.Citation28 The wounding of leaf explants with the sterile fine needle was necessary for inducing transformation in case of tobacco. In case of tea the injury of somatic embryos result lower number of transformation events and tissue browning.Citation29 Similar reports were found in case of walnut.Citation30 AS, a potent vir gene inducer, was initially shown to enhance the transformation efficiency of Agrobacterium in A. thalianaCitation31 and Glycine max.Citation32 In the present study we have used 150 mM AS concentration. In case of poplars the AS suppress transformation frequency.Citation33
Figure 2.Agrobacterium-mediated genetic transformation of four genotypes of Nicotiana. (A) Pre culture of explants; (B) co-cultivation; (C) pre-selection; (D) selection; (E) maturation of cotyledonary stage embryos; (F) elongation and rooting; (G) hardening in green house.
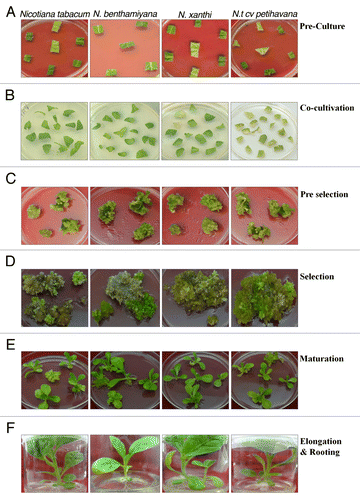
In several plant species, co-cultivation for 2–7 d has been found to be suitable for Agrobacterium-mediated transformation. High-frequency transformation was obtained by co-cultivating () tobacco explants, with the disarmed strain (LBA 4404), for 3 d. Co-cultivation for > 3 d led to a decrease in transformation frequency. The transformation efficiency is known to be influenced by the Agrobacterium strain used as well as the co-cultivation medium. Also, effect of the co-cultivation medium’s pH has been extensively studied. An acidic pH of 5.5 has been found to effectively induce the vir (virulence) genes Citation34,Citation35. In another study performed using five different strains of Agrobacterium, pH of 5.6 was found to be most efficient in causing maximum tumorigenicity in potato leaf discs.Citation36 In our study, however, we obtained maximum transformation efficiency using co-cultivation medium of pH 5.4. It could be argued that an increased time of incubation could have positive influence on of transformation rate, but our study contradicts the statement, as explants showed extensive damage after 40 min of incubation. The agro-infected explants were further transferred into pre-selection medium (). To inhibit Agrobacterium growth, the pre-selection medium was supplemented with 250 mg/l cefatoxime. Later the preselected explants were subjected to 3 rounds of selection medium to select transformed cells () with one week interval. The putative transgenic somatic embryos that thrived on the selection medium developed, through the normal stages of embryogenesis (globular, heart-shape, torpedo-shaped stages) (), into green and healthy cotyledonary-staged embryos. These embryos germinated into plantlet in the hygromycin supplemented selection media with proper rotting and morphological character (). The well rooted plants were further transferred to pots where they exhibited healthy growth, normal flowering and seed set ().
Confirmation of gene integration in the transgenics
The presence and the expression of the transgene was verified utilizing molecular analysis including histochemical GUS analysis and PCR using GUS gene specific primers. A successful transformation event has earlier been verified by PCR analysis in peach tissues.Citation37-Citation39 Earlier the integration of the GUS gene in the banana genome was also reported to be confirmed by PCR.Citation40–Citation42 The PCR analysis, performed using total genomic DNA from transgenic as well as the WT tissues (non-transformed), yielded an expected amplification of 500 bp using GUS gene specific primers in the transformed plants which was absent in the WT control () indicating presence of the GUS gene in the transgenic plants.
Figure 3. Histochemical and molecular confirmation of tobacco transgenics. (A) PCR analysis of primary transformants using GUS gene specific primers. L1, line 1; L2, line 2; L3, line 3; WT, wild type control (non-transgenic); PC, positive control (vector pCAMBIA1301). (B) Stable GUS activity in the tobacco transgenics of all the four genotypes.
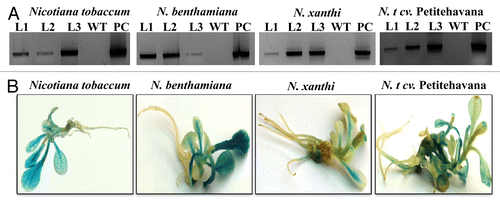
5-bromo-4-chloro-3-indol glucuronide (X-gluc) serves as an efficient substrate for β-glucuronidase. It is proven as an efficient method to monitor integration of the β-glucuronidase gene in tissue and cells and is used as the reporter gene in the binary vector employed in plant transformation. In our study we performed histochemical GUS assay on the hygromycin resistant transformed tobacco tissues.Citation43 The stable GUS activity was checked five weeks after co-cultivating plantlets of all the four transgenic tobacco genotypes with Agrobacterium. The results showed a positive GUS expression in all the tested plant lets, which confirmed the successful integration as well as expression of the transgene ().
Conclusion
We report a highly efficient and reproducible regeneration system without an intervening callus phase based on direct SE for four genotypes of Nicotiana. The somatic embryos were initiated from the leaf explants in presence of BAP and IAA but their further maturation was achieved in hormone free MS medium. In addition we have tested this regeneration system for successful Agrobacterium-mediated transformation, in all the four genotypes of tobacco, using leaf as explant. The plantlets that successfully survived on hygromycin selection were analyzed for stable gene integration using PCR analysis which was cross-verified using GUS histochemical assay. The plant regeneration and transformation method optimized in the current study presents a simple, efficient and rapid method for the introduction of a transgene directly in tobacco through using SE. Also, the time required to generate plantlets from a leaf pre-culture was shortened to about 40–45 d. The established protocol would also contribute to large-scale vegetative propagation, germplasm conservation and transformation-mediated genetic improvement analysis.
Materials and Methods
Seed sterilization and germination
Healthy viable seeds of four different cultivars of tobacco (Ncotiana tabacum L, N. benthamiyana, N.t petithavana, N. xanthl) were surface-sterilized by rinsing in 70% ethanol for 45 sec and then washed three times in sterile distilled water. Seeds were further disinfected with 0.1% mercuric chloride solution for 45 sec. The sterilant was removed by washing the seeds several times with sterile distilled water then the seeds were blotted dry in Whatman filter paper and the seeds were placed in a Petri dish containing seed germination medium. The seeds were maintained culture room conditions. After 30 d plants with roots were transferred to jam bottles containing same media.
Preparation and culture of explants
For all the experiments including transformation 45 d old first pairs of fully expanded leaves were used. The leaves were trimmed in 1 × 1 mm pieces and aseptically placed with the abaxial surface touching the culture medium.
Culture media and growth conditions
MSCitation44 with B5 vitamins, 2% (w/v) sucrose, 0.03% (w/v) phytagel was used in all the experiments. The pH of the medium was adjusted to 5.8 by 1 M NaOH or 1 M HCl before autoclaving at 121°C for 20 min. All plant growth regulators were added after autoclaving the medium. The cultures were incubated at 25 ± 2°C in a culture room with 50 μmol m−2 s−1 irradiance provided by cool fluorescent lamps and were exposed to a photoperiod of 16 h and 55% relative humidity.
Somatic embryo induction and maturation
For the induction of somatic embryo leaf explants were aseptically placed on MS medium with B5 vitamins supplemented with different concentrations of IAA (0.1–0.25 mg/l), BAP (1.5–3 mg/l) with 20 g/l sucrose In order to determine the best concentration to produce direct SE. Cotyledonary stage embryos were transferred to ms medium supplemented with 30 g/l sucrose. Cultures were incubated under the same environmental conditions used for shoot elongation and as well as rooting. Plantlets produced from the rooting stage were transferred from the Jam bottles to pots containing peat moss and sand (1:1). Plastic pots were enveloped in polyethylene bags for few days. Thereafter, bags were removed and maintained under greenhouse conditions.
Agrobacterium-mediated transformation protocol
The transformation of four varieties (Nicotiana tabacum, N. benthamiyana, N. xanthi, N.t cv petihavana ) were done by Agrobacterium-mediated genetic transformation. The disarmed A. tumefacies strain LBA4404, harbouring the binary vector pCAMBIA1301, containing the β glucuronidase (GUS) gene and a hygromycin phosphotransferase gene (hpt), both driven by the cauliflower mosaic virus (CaMV) 35S promoter, was used for transformation. The GUS gene contains an intron in its coding region, in order to ensure that the observed GUS activity occurs in the plant cells and not due to the presence of residual Agrobacterium cells. The disarmed A. tumefacies strain LBA 4404 (harboring the binary vector pCAMBIA1301) was grown on YEM solid medium containing 50 mg of Kanamycin and 10 mg Rifampicin at 28°C. A single bacterial colony was inoculated into 3 ml of YEM (primary culture) containing the same antibiotics and grown over night on a rotary shaker at 180 rpm at 28°C. An aliquot of 1 ml bacterial suspension was added to 100 ml YEM medium with the additional antibiotics and grown over night. Bacteria were pelleted at 4000 rpm for 5 min and resuspended in liquid MS medium with 200 μM acetosyringone (AS) at a density of 0.8 OD at 600 nm. Re-suspended bacterial cells were shaken (175 rpm) at 28°C for 1 h before use. Pre cultured leaf explants (0.5–1 cm) were collected in sterile Petri dishes under aseptic conditions. Explants were immersed in resuspended Agrobacterium culture for 25–30 min with continuous shaking. The Agrobacterium-treated explants were then blotted on sterile filter paper and transfer to co-culture medium containing 0.8% agar, for 3 d in the dark at 23–25°C. After co-cultivation leaf explants were planted on pre selection medium later these explants were transferred to regeneration medium containing 250 mg/l cefotaxime 30 mg/l hygromycine was added to select the transformed cells whereas the cefotaxim antibiotic was added to inhibit Agrobacterium growth. The cultures were kept in the growth chamber at 26 ± 2°C under 16 h photoperiod of 3000 Lux supplied with cool white fluorescent lamps. Following a four week culture period, with subculturing to fresh medium at eight-day intervals, hygromycine resistant somatic embryos were developed .These embryos were further subcultured in the same regeneration medium for further development. After 3 rounds of selections cotyledonary stage embryos were transferred to the elongation medium they were further rooted in the same medium.
Histochemical assay for the GUS activity
The histochemical assay for the expression of GUS gene (β-D-Glucuronidase) was performed on the transformed plants by using 5-Bromo-4-chloro-3-indolyl Glucuronide (X-Gluc) as a substrate, as indicated by the established method.Citation43 The putative transformed plantlets were kept in sodium phosphate buffer (50 mM NaPO4, pH6.8) that contain 1% Triton X-100 at 37°C for 1 h and later on, were incubated overnight in a solution containing 1.0 mM × -Gluc 10 mM EDTA, 100 mM NaH2PO4, 0.1× TritonX-100 and 50% methnol (pH 5.8). The tissues were washed twice in 99% methanol for 2 h to remove chlorophyll pigment. GUS expression was visually observed and photographed by using sony nex-3 camera.
PCR analysis of GUS gene
Samples were collected from four varieties of putative transgenic tobacco plants as well as the corresponding control (non-transformed plants) and subjected to DNA extraction by using CTAB method. The forward and reverse primer sequences (F-5′-GAGGCT AAT TCG GCT ATG ACT G-3′ and, R-5′-ATCGGG AGA GGC GAT ACC GTA-3′ respectively)(Eurofins India) used for PCR amplification of the GUS gene. The primers were so designed as to give amplification products of the internal sequence of the GUS of 500 bp. The PCR mixture (25 μl) contained 1.5 U Taq DNA polymerase, 10 mM TRIS-HCl (pH = 9.0), 50 mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP, 1 μl of each forward and reverse primer at a final concentration of 10 pmol and 50 ng template DNA. 50 pg of pCAMBIA1301 plasmid DNA was used as a positive control. Samples for enzymatic amplification were subjected to an initial program of one cycle of 95°C for 180 sec followed by 35 cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for 60 sec. Finally, the reaction mixtures were allowed to complete an additional extension of 10 min at 72°C before rapid cooling to 4°C. The PCR was performed using a thermal cycler bio-rad (Model T100) with the fastest available transitions between temperatures. The amplification products were separated by 1% agarose gel electrophoresis with ethidium bromide solution (0.5 μg/ml) in 1× TAE buffer (0.04 M TRIS-acetate, 1 mM EDTA, pH = 8) at 80 V for 45 min and the gel was photographed under an Alpha innotech Doc System.
| Abbreviations: | ||
| BAP | = | 6-benzylaminopurine |
| IAA | = | indole-3-acetic acid |
| PGR | = | plant growth regulator |
| TDZ | = | thidiazuron |
| AS | = | acetosyringone |
Acknowledgments
Work on plant transformation and plant abiotic stress tolerance in N.T.’s laboratory is partially supported by Department of Biotechnology (DBT) and Department of Science and Technology (DST), Government of India. We thank Dr Renu Tuteja and Miss Neha Vaid for their useful suggestions in editing the manuscript, Dr Praveen Verma (NIPGR, New Delhi) for providing microscopic facility and Mr Kazi Md. Kamrul Huda for his help to analyze the results.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun 2005; 73:8266 - 74; http://dx.doi.org/10.1128/IAI.73.12.8266-8274.2005; PMID: 16299323
- Ammirato PV. Hand Book of Plant Cell Culture Evans. In: D. A. et al. (Eds.) Macmillan, New York, 1983; 1:82–123.
- Sato S, Newell C, Kolacz K, Tredo L, Finer J, Hinchee M. Stable transformation via particle bombardment in two different soyabean regeneration system. Plant Cell Rep 1993; 12:408 - 13; http://dx.doi.org/10.1007/BF00234702
- Parrott WA, Dryden G, Vogt S, Hildebrand DF, Collins GB, Williams EG. Optimization of somatic embryogenesis and embryo germination in soybean. In vitro Cell Dec. Biol 1988; 24:817 - 20
- Slater A, Scott NW, Fowler MR. Genetic manipulation of plants. Plant Biotechnology Oxford, England: Oxford University Press 2003; 350pp.
- Zimmerman JL. Somatic embryogenesis: a model for early development in higher plants. Plant Cell 1993; 5:1411 - 23; PMID: 12271037
- Bandyopadhyay S, Hamill JD. Ultra structural studies of somatic embryos of Eucalyptus nitens and comparisons with zygotic embryos found in mature seeds. Ann Bot (Lond) 2000; 86:237 - 44; http://dx.doi.org/10.1006/anbo.2000.1192
- Saiprasad GVS. Artificial seeds and their applications. Resonance 2001; 6:39 - 47; http://dx.doi.org/10.1007/BF02839082
- Jimenez VM. Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. Rev Bras Fisiol Vegetal 2001; 13:196 - 223; http://dx.doi.org/10.1590/S0103-31312001000200008
- Singh B, Sharma S, Rani G. In vitro response of encapsulated and non-encapsulated somatic embryos of Kinnow mandarin (Citrus nobilis Lour x C. deliciosa Tenora). Plant Biotechnol Rep 2007; 1:101 - 7; http://dx.doi.org/10.1007/s11816-007-0015-6
- Stolarz A, Macewicz J, Lorz H. Direct somatic embryogenesis and plant regeneration from leaf explants of Nicotiana tabacum L.. J Plant Physiol 1991; 137:347 - 57; http://dx.doi.org/10.1016/S0176-1617(11)80144-6
- Gill R, Saxena PK. Somatic embryogenesis in Nicotiana tabacum L. induction by TDZ of direct embryo differentiation from cultured leaf discs. Plant Cell Rep 1993; 12:154 - 9; http://dx.doi.org/10.1007/BF00239097
- Huetteman CA, Preece JE. TDZ: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 1993; 33:105 - 19; http://dx.doi.org/10.1007/BF01983223
- Lu CY. The use of TDZ in tissue culture. In Vitro Cell Dev Biol 1993; 29P:92 - 6
- Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 1957; 11:118 - 30; PMID: 13486467
- Prabhudesai VR, Narayanswamy S. Differentiation of cytokine ininduced shoot buds and embryoids on excised petioles of Nicotiana tabacum.. Phytomorphology 1974; 23:133 - 7
- Brown DCW, Thorpe TA. Plant regeneration by organogenesis.In: Cell culture and somatic cell genetics of plants OK Vasil (Eds.) Academic Press. Orlando, Florida 1986; 49-65.
- Haccius B, Lakshmanan KK. Adventive embryony in callus culture of Nicotiana under high light intensity. Planta 1965; 65:102 - 4; http://dx.doi.org/10.1007/BF00385183
- Sunilkumar G, Vijayachandra K, Veluthambi K. Preincubation of cut tobacco leaf explants promotes Agrobacterium-mediated transformation by increasing vir gene induction. Plant Sci 1999; 141:51 - 8; http://dx.doi.org/10.1016/S0168-9452(98)00228-3
- Schmidt R, Willmitzer L. High efficiency Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana leaf and cotyledon explants. Plant Cell Rep 1988; 7:583 - 6; http://dx.doi.org/10.1007/BF00272763
- Sangwan RS, Bourgeois Y, Brown S, Vasseur G, Sangwan-Norreel BS. Characterization of competent cells and early events of Agrobacterium-mediated genetic transformation of Arabidopsis thaliana. Planta 1992; 188:439 - 56; http://dx.doi.org/10.1007/BF00192812
- Fillatti JJ, Kiser J, Rose R, Comai L. Efficient transfer of a glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Biotechnology 1987; 5:726 - 30; http://dx.doi.org/10.1038/nbt0787-726
- Sangwan RS, Ducrocq C, Sangwan-Norreel BS. Effect of culture conditions on Agrobacterium-mediated transformation in datura. Plant Cell Rep 1991; 10:90 - 3; http://dx.doi.org/10.1007/BF00236464
- Jacq B, Lesobre O, Sangwan RS, Sangwan-Norreel BS. Factors influencing T-DNA transfer in Agrobacterium mediated transformation of sugarbeet. Plant Cell Rep 1993; 12:621 - 4; http://dx.doi.org/10.1007/BF00232811
- Choi PS, Soh WY, Kim YS, Yoo OJ, Liu JR. Genetic transformation and plant regeneration of watermelon using Agrobacterium tumefaciens.. Plant Cell Rep 1994; 13:344 - 8; http://dx.doi.org/10.1007/BF00232634
- Confalonieri M, Balestrazzi A, Bisoffi S. Genetic transformationof Populus nigra by Agrobacterium tumefaciens.. Plant Cell Rep 1994; 13:256 - 61; http://dx.doi.org/10.1007/BF00233315
- Janssen B, Gardner RC. The use of transient GUS expressionto develop an Agrobacterium-mediated gene transfer system for kiwifruit. Plant Cell Rep 1993; 13:18 - 31; http://dx.doi.org/10.1007/BF00232310
- Archilletti T, Lauri P, Damiano C. Agrobacterium-mediated transformation of almond leaf pieces. Plant Cell Rep 1995; 14:267 - 72; http://dx.doi.org/10.1007/BF00232026
- Mondal TK, Bhattacharya A, Ahuja PS, Chand PK. Transgenic tea [Camellia sinensis (L.) O. Kuntze cv. Kangra Jat] plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep 2001; 20:712 - 20; http://dx.doi.org/10.1007/s002990100382
- McGranahan GH, Leslie CA, Uratsu SL, Martin LA, Dandekar AM. Agrobacterium-mediated transformation of walnut somatic embryos and regeneration of transgenic plants. Biotechnology 1988; 6:800 - 4; http://dx.doi.org/10.1038/nbt0788-800
- Sheikholeslam SN, Weeks DP. Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens.. Plant Mol Biol 1987; 8:291 - 8; http://dx.doi.org/10.1007/BF00021308
- Owens LD, Smigocki AC. Transformation of soybean cells using mixed strains of Agrobacterium tumefaciens and phenolic compounds. Plant Physiol 1988; 88:570 - 3; http://dx.doi.org/10.1104/pp.88.3.570; PMID: 16666350
- De Kathen A, Jacobsen HJ. Agrobacterium tumefaciens mediated transformation of Pisum sativum L. using binary and cointegrate vectors. Plant Cell Rep 1990; 9:276 - 9; http://dx.doi.org/10.1007/BF00232301
- Stachel SE, Nester EW, Zambryski PC. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci USA 1986; 83:379 - 83; http://dx.doi.org/10.1073/pnas.83.2.379; PMID: 16593648
- Alt-Moerbe J, Neddemann P, Von Lintig J, Weiler EW, Schroder J. Temperature sensitive step in Ti plasmid vir region induction and correlation with cytokinin secretion by Agrobacterium.. Mol Gen Genet 1988; 213:1 - 8; http://dx.doi.org/10.1007/BF00333390
- Boudijeniba M, Hunault G. The potato disc bioassay. Effect of some parameters related to the inoculum and to the growth medium on the development of tumers. Ann Sci Nat Bot Biol Veg 1989; 10:134 - 48
- Xiaojian YE, Scorza R, Sanford JC. Genetic transformation of peach tissues by particle bombardment. J Am Soc Hortic Sci 1994; 119:367 - 73
- Smigocki AC, Hammerschlag FA. Regeneration of plants from peach embryo cells infected with a shooty mutant strain of Agrobacterium.. J Am Soc Hortic Sci 1991; 116:1092 - 7
- Gonzalez Padilla IM, Webb K, Scorza R. Early antibiotic selection and efficient rooting and acclimatization improve the production of transgenic plum plants (Prunus domestica L.). Plant Cell Rep 2003; 22:38 - 45; http://dx.doi.org/10.1007/s00299-003-0648-z; PMID: 12827433
- Sagi L, Panis B, Remy S, Schoofs H, De Smet K, Swennen R, et al. Genetic transformation of banana and plantation (Musa spp.) via particle bombardment. Bio-Tech 1995; 13:481 - 5; http://dx.doi.org/10.1038/nbt0595-481
- Dugdale B, Beetham PR, Becker DK, Harding RM, Dale JL. Promoter activity associated with the intergenic regions of banana bunchy top virus DNA-1 to -6 in transgenic tobacco and banana cells. J Gen Virol 1998; 79:2301 - 11; PMID: 9780033
- Becker DR, Dugdale B, Smith MK, Harding RM, Dale JL. Genetic transformation of Cavendish banana (Musa spp. AAA group) cv. Grand Nain via microprojectile bombardment. Plant Cell Rep 2000; 19:229 - 34; http://dx.doi.org/10.1007/s002990050004
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 1987; 6:3901 - 7; PMID: 3327686
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962; 15:473 - 97; http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x