Abstract
Natural cytokinins as well as the majority of their synthetic derivatives show negative effects on root growth and development. Changes in morphology, primarily linked to the inhibition of the cell division in the meristematic zone, are manifested as thickening and shortening of the primary root and impaired lateral root branching. Rational design of cytokinin derivatives can partially overcome these drawbacks and reduce the negative effects. Using our database of cytokinin derivatives, we selected several aromatic cytokinin analogs with modifications at the N9 atom of the adenine moiety. We found that tetrahydropyranyl and tetrahydrofuranyl substitutions at the N9 atom led to enhanced acropetal transport of the modified cytokinin, and both derivatives also showed weak anticytokinin activity. Consequently, changes in the distribution of the active cytokinin pool together with gradual metabolic conversion of the modified cytokinin to its free form prevent root growth inhibition that limits cytokinin utilization in micropropagation techniques.
Purine-based cytokinins (CKs) are naturally occurring plant hormones responsible for the regulation of various developmental processes such as coordination of cell division, cell proliferation, differentiation of roots and leaves or seed germination.Citation1-Citation3 CKs are also indispensable in plant micropropagation techniques, where they promote cell division and secure normal and proportional organ development. The micropropagation accompanied with CK application into culture media is a rapid biotechnological method that has been adopted for commercialization of important crop plants such as banana,Citation4 apple,Citation5 rose,Citation6 strawberry,Citation7 potatoes,Citation8 as well as for the preservation of endangered species such as Harpagophytum prucubens or Aloe polyphylla.Citation9 In micropropagation, N6-furfuryladenine (kinetin), N6-isopentenyladenine (iP), trans-zeatin (tZ) and N6-benzylaminopurine (BAP) are the most frequently used CKs for callus re-differentiation into adventitious buds. CKs with an isoprenoid side-chain (iP, tZ) have one major disadvantage when compared with aromatic CKs—they are susceptible to fast oxidative degradation. On the contrary, both kinetin and BAP are more stable in vivo,Citation10 and show anti-senescence and anti-stress properties, which may be related to their ability to prevent formation of reactive oxygen species.Citation11 Although BAP is currently used as the most common and affordable aromatic CK in tissue culture propagations, its utilization has several drawbacks such as shoot-tip necrosis, inhibition of rooting and problematic acclimatization of plant in the greenhouse.Citation12 It has been hypothesized that some of these negative effects would be caused by natural N9-glucosylation of the applied purine-based CK, leading to extensive accumulation of non-active CK glucosides.Citation13 Alternatively, we have recently suggested that the N9-glucosides would activate ethylene production and its signaling pathway causing the inhibition of root elongation.Citation14 The adverse effects of N9-glucosylation and thus of aromatic CK treatment could be suppressed by appropriate N9 purine substitution of BAP or methoxylation of its benzyl ring.
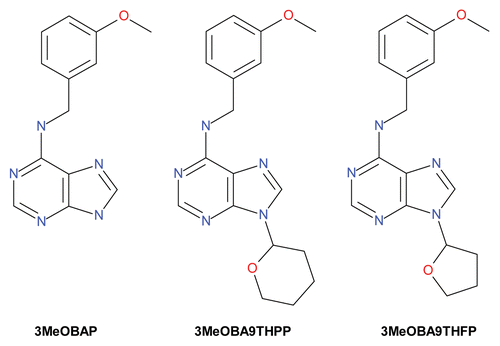
In our previous work, to test this hypothesis, we selected 3MeOBAP over non-modified BAP due to its higher stability and activity caused by the fact that it is less prone to CK degradation by catabolic enzymes.Citation10 We designed a small library of N9-derivatives of 3MeOBAP using ribose (yielding 3MeOBAPR), tetrahydropyran-2-yl (yielding 3MeOBA9THPP, ) and 4-chlorobutyl (yielding 3MeOBA9ClButP) as substituents and tested their activity in standardized CK activity assays.Citation10,Citation14 Although the substitution at the N9 position very often decreases the CK activity, this was not the case for the 3MeOBAP derivatives. Then, we compared the effect of treatment with 50 nM 3MeOBAP to that of its three derivatives on the morphology of germinating Arabidopsis and maize seedlings. For Arabidopsis seedlings grown on MS media supplemented with 3MeOBA9THPP, 3MeOBA9ClButP or the N9-glucoside, 3MeOBAP9G, we did not observe negative effects on root elongation in a broad range of concentrations (from 0.04 to 1 µM). Moreover, 3MeOBA9ClButP even showed a distinct positive effect on lateral root formation. In contrast, for maize 3MeOBA9ClButP and 3MeOBAP9G had a slightly negative effect on root proliferation in the submicromolar concentration range (8–40 nM). On the other hand, 3MeOBA9THPP in the same concentration range, showed no negative effect on primary root elongation and, in addition, had a positive impact on the growth of the aerial part when compared with 3MeOBAP.Citation14
Here, we tested another 3MeOBAP N9 atom substituent —tetrahydrofuran-2-yl (yielding 3MeOBA9THFP, )—selected from the oxygen heterocyclic family. When selected CKs were applied into the MS media, we could observe characteristic effects on root elongation (). Similarly to our previous results,Citation14 the application of 50 nM 3MeOBA9THPP had positive effect on primary root elongation when compared with untreated controls (cp. primary root lengths: 11.4 ± 1.2 cm vs. 9.9 ± 0.6 cm). The application of 50 nM 3MeOBA9THFP had a low inhibitory effect when compared with the strong effect of 3MeOBAP/3MeOBAPR (cp. primary root lengths: 9.4 ± 1.1 cm vs. 4.0 ± 0.3 cm and 6.5 ± 0.6 cm, for 3MeOBAP and 3MeOBAPR, respectively). Just like 3MeOBA9THPP, 3MeOBA9THFP had a distinct positive effect on aerial plant development ().
Figure 2. Maize seedlings cv CELLUX cultivated on MS agar in magenta boxes treated with 50 nM 3MeOBAP (B) and its derivatives: 3MeOBAPR (C), 3MeOBA9THFP (D) and 3MeOBAPTHPP (E) in comparison to DMSO-treated control plant (A). Seedlings were germinated for three days, transferred to MS agar and grown at 24°C under long-day conditions for another seven days prior to taking pictures. Representative plants selected from six independent treatments are shown.

The observed properties of 3MeOBA9THPP and by extrapolation of 3MeOBA9THFP can be explained on the basis of their enhanced translocation from root to leaf as well as by the specific enzymatic conversion that is necessary for their activation. Indeed, we found that acropetal transport of 3MeOBA9THPP was significantly strengthened when compared with free base transport.Citation14 Moreover, when roots were treated with 3MeOBA9THPP, the total 3MeOBAP CK pool in the xylem sap was about 10 times higher than that in control plants treated with the free base. This unexpected finding suggested that the concentration of active CKs in the aerial part of the plants would probably be higher in case of 3MeOBA9THPP treatment, explaining the observed positive effect on the maize shoot. Our subsequent detailed analysis of the endogenous CK pool in plants treated with 3MeOBA9THPP or 3MeOBAP confirmed that the distribution of CKs in these two treatments showed important changes.Citation14 Additionally, we explored the intracellular faith of the CK analogs in maize using tritium labeled 3MeOBA9THPP-treated samples purified and fractionated on a C18 separation column.Citation14 We found that the THP group can be cleaved off from its parent CK both in the root and the leaf. Subsequently, the free base can be glycosylated at the N9 position to provide the glucoside or riboside. Moreover, 3MeOBAP can also be de-methylated and the resulting 3OHBAP and its glucosides are found especially in the leaves of treated plants. In plants treated with 3MeOBAP, however, the most predominant metabolic product we detected was its N9-glucoside (either in the 3-OH methylated or de-methylated form).Citation14 Then, we analyzed the endogenous CK content in maize roots and leaves treated or not for three days with either 3MeOBAP or 3MeOBA9THPP. We found that whereas the endogenous pool of tZ and iP type CKs remained largely unchanged in 3MeOBA9THPP-treated maize leaves, upon treatment with 3MeOBAP their level significantly decreased. cZ and DHZ levels did not change in response to both treatments. Hence, the positive effect of 3MeOBA9THPP treatment on plant development is correlated with the endogenous CK levels, strongly suggesting that the observed phenotypic effects are proportional to the concentration of the active CK forms, which result from the metabolic conversions of exogenously applied CK and from successful homeostasis of endogenous isoprenoid CKs.Citation14
To address the question of biological activity of a given CK form, we can employ two types of biological assays, both of which are based on heterologous expression of the plant CK histidine kinase (HK) receptor in E. coli strain KMI001. The receptor activation test reflects the ability of the ligand to initiate signal transduction from the tested HK receptor and makes use of the reporter gene lacZ under the control of cps promoter.Citation15 β-galactosidase activity was assayed using the fluorogenic substrate 4-methylumbelliferyl-β-D-galactopyranoside after overnight incubation of the bacteria with selected CKs. As previously reported, none of the studied N9-analogs were able to activate the Arabidopsis or maize HK receptors,Citation14 which is in agreement with the general view on N9-modified CKs as biologically non-active.
Here, to further explore the biological roles of the prepared N9-modified CKs, we measured the interaction of the ligand with the HK receptor activation site using a receptor competition test reasoning that even when the ligand cannot initiate signal transduction, it is still possible that it enters the receptor activation site. Such an interaction is biologically relevant, because a group of compounds, referred to as anticytokinins, can competitively inhibit binding of the natural substrate to the target CK receptorCitation16 and, therefore, anticytokinins are important players in the intracellular CK signaling pathways. Importantly, it has been reported that anticytokinin PI-55 accelerated the germination of Arabidopsis seeds and promoted the root growth and formation of lateral roots.Citation16As such, we assessed whether 3MeOBA9THPP, 3MeOBA9THFP and 3MeOBAP were able to compete with tritium-labeled tZ in the receptor activation site of the Arabidopsis receptor AHK4 (). Surprisingly, both 3MeOBA9THPP and 3MeOBA9THFP showed a moderate ability to interact with the receptor activation site. Even more, in the higher concentration range for 3MeOBA9THFP, a significant competition comparable to that of 3MeOBAP was observed. Thus, some CK N9-derivatives appear to partially retain their biological activity and affect the CK concentration in situ in the receptor binding site in a similar way as anticytokinins.
Figure 3. Competitive receptor test of selected CKs with 2 nM [3HtZ] for binding into the Arabidopsis CK receptor AHK4 using a bacterial competition assay.Citation15,Citation16 The assay was done with different concentrations of the CKs, tZ served as a positive and adenine (Ade) as a negative control for ligand binding. The values are expressed as means ± SD of three independent replicates.
![Figure 3. Competitive receptor test of selected CKs with 2 nM [3HtZ] for binding into the Arabidopsis CK receptor AHK4 using a bacterial competition assay.Citation15,Citation16 The assay was done with different concentrations of the CKs, tZ served as a positive and adenine (Ade) as a negative control for ligand binding. The values are expressed as means ± SD of three independent replicates.](/cms/asset/844257c3-24ad-4b20-bcb4-3aa8e466598d/kpsb_a_10924392_f0003.gif)
In conclusion, our data show that some of the N9-derivatives of CKs may have important biological functions. Although these derivatives seem to be lacking the ability to switch on the HK receptor, possibly they can still partially interact with the receptor activation site and compete for binding with endogenous CKs. Despite the general view on N9-analogs as terminal metabolic products, we have gathered clear evidence that some derivatives, such as 3MeOBA9THPP and 3MeOBA9THFP, can be effectively processed in maize, resulting in the free base which is then involved in CK-mediated signaling. Importantly, enhanced acropetal transport of these derivatives is another regulatory mechanism that allows uniform distribution of CKs within the plant body and secure good organ development. Thus, these novel N9-substituted derivatives of the aromatic CKs have a great potential as alternative compounds to improve plant in vitro regeneration techniques.
| Abbreviations: | ||
| BAP | = | N6-benzylaminopurine |
| CK | = | cytokinin |
| 3MeOBAP | = | 3-methoxy-6- (benzylamino)purine |
| 3MeOBAPR | = | 3-methoxy-6-(benzylamino)purine riboside |
| 3MeOBA9THPP | = | 3-methoxy-6-benzylamino-9-(tetrahydropyran-2-yl)purine |
| 3MeOBA9THFP | = | 3-methoxy-6-benzylamino-9-(tetrahydrofuran-2-yl)purine |
| cZ | = | cis-zeatin |
| tZ | = | trans-zeatin |
| iP | = | N6-isopentenyladenine |
Acknowledgments
We would like to thank Martin Tkáč and Aneta Wanderlová for their technical assistance. This work was supported by grants GAP522/10/1141 and GAP501/10/1450 from the Czech Science Foundation and COST project LD12061.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 2001; 98:10487 - 92; http://dx.doi.org/10.1073/pnas.171304098; PMID: 11504909
- Mok DW, Mok MC. Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:89 - 118; http://dx.doi.org/10.1146/annurev.arplant.52.1.89; PMID: 11337393
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003; 15:2532 - 50; http://dx.doi.org/10.1105/tpc.014928; PMID: 14555694
- Muhammad A, Rashid H, Hussain I. Proliferation-rate effects of BAP and kinetin on banana (Musa spp. AAA Group) ‘Basrai’. HortScience 2007; 42:1253 - 5
- Dobránszki J, da Silva JA. Micropropagation of apple--a review. Biotechnol Adv 2010; 28:462 - 88; http://dx.doi.org/10.1016/j.biotechadv.2010.02.008; PMID: 20188809
- Pati PK, Rath SP, Sharma M, Sood A, Ahuja PS. In vitro propagation of rose--a review. Biotechnol Adv 2006; 24:94 - 114; http://dx.doi.org/10.1016/j.biotechadv.2005.07.001; PMID: 16169183
- Borkowska B. Morphological and physiological characteristics of micropropagated strawberry plants rooted in vitro or ex vitro. Sci Hortic (Amsterdam) 2001; 89:195 - 206; http://dx.doi.org/10.1016/S0304-4238(00)00230-2
- Baroja-Fernández E, Aguirreolea J, Martínková H, Hanuš J, Strnad M. Aromatic cytokinins in micropropagated potato plants. Plant Physiol Biochem 2002; 40:217 - 24; http://dx.doi.org/10.1016/S0981-9428(02)01362-1
- Bairu MW, Jain N, Stirk WA, Doležal K, Van Staden J. Solving the problem of shoot-tip necrosis in Harpagophytum procumbens by changing the cytokinin types, calcium and boron concentrations in the medium. S Afr J Bot 2009; 75:122 - 7; http://dx.doi.org/10.1016/j.sajb.2008.08.006
- Szücová L, Spíchal L, Doležal K, Zatloukal M, Greplová J, Galuszka P, et al. Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives. Bioorg Med Chem 2009; 17:1938 - 47; http://dx.doi.org/10.1016/j.bmc.2009.01.041; PMID: 19232496
- Mik V, Szüčová L, Smehilová M, Zatloukal M, Doležal K, Nisler J, et al. N9-substituted derivatives of kinetin: effective anti-senescence agents. Phytochemistry 2011; 72:821 - 31; http://dx.doi.org/10.1016/j.phytochem.2011.02.002; PMID: 21354583
- Werbrouck SPO, Strnad M, Van Onckelen H, Debergh PC. Meta-topolin, an alternative to benzyladenine in tissue culture?. Physiol Plant 1996; 98:291 - 7; http://dx.doi.org/10.1034/j.1399-3054.1996.980210.x
- Werbrouck SPO, van der Jeugt B, Dewitte W, Prinsen E, van Onckelen HA. The metabolism of benzyladenine in Spathiphyllum floribundum ‘Schott Petite’ in relation to acclimatisation problems. Plant Cell Rep 1995; 14:662 - 5; http://dx.doi.org/10.1007/BF00232734
- Podlešáková K, Zalabák D, Cudejková M, Plíhal O, Szüčová L, Doležal K, et al. Novel cytokinin derivatives do not show negative effects on root growth and proliferation in submicromolar range. PLoS ONE 2012; 7:e39293; http://dx.doi.org/10.1371/journal.pone.0039293; PMID: 22723989
- Suzuki T, Miwa K, Ishikawa K, Yamada H, Aiba H, Mizuno T. The Arabidopsis sensor His-kinase, AHk4, can respond to cytokinins. Plant Cell Physiol 2001; 42:107 - 13; http://dx.doi.org/10.1093/pcp/pce037; PMID: 11230563
- Spíchal L, Werner T, Popa I, Riefler M, Schmülling T, Strnad M. The purine derivative PI-55 blocks cytokinin action via receptor inhibition. FEBS J 2009; 276:244 - 53; http://dx.doi.org/10.1111/j.1742-4658.2008.06777.x; PMID: 19032596