Abstract
The optimal defense hypothesis (ODH) provides a functional explanation for the inhomogeneous distribution of defensive structures and defense metabolites throughout a plant’s body: tissues that are most valuable in terms of fitness and have the highest probability of attack are generally the best defended. In a previous review,Citation1 we argue that ontogenically-controlled accumulations of defense metabolites are likely regulated through an integration of developmental and defense signaling pathways. In this addendum, we extend the discussion of ODH patterns by including the recent discoveries of circadian clock-controlled defenses in plants.
Optimal Defense Predictions
Differential accumulation of plant defenses, for example their higher levels in younger sink tissues compared with other parts, have long been recognized and have inspired various hypotheses that invoke developmental, ecological and evolutionary constraints,Citation2 with the ODH as one of the most influential.Citation3 This hypothesis includes the assumptions that: (1) plants use their limited resources to preferentially direct defenses to those tissues that are (2) comparably more likely to be attacked by herbivores and pathogens and (3) more valuable than others. While the ODH mainly addresses differences in defensive states among distinct tissues of a plant or their change during the vegetative growth, the short-term diurnal fluctuations in defense regimes that were recently found to occur have yet to be considered in light of the ODH.
Circadian Control of Plant Defenses
The circadian clock enables plants to synchronize their overall physiology to predictable day-night regimes in their environment. Thereby, plants can adjust their metabolism to diurnal oscillations such as differences in temperature or light.Citation4-Citation6 In Arabidopsis thaliana, about 30% of the plant’s transcriptome is regulated by the circadian clock;Citation7 including transcripts of genes belonging to well-known defense pathways. Some of these circadian-controlled defense pathways are regulated by jasmonic acid (JA), which plays important roles in mediating plant defense against herbivores.Citation1,Citation8 Indeed, basal levels of JA in leaves were shown to follow a sharp diurnal rhythm, with a peak at the middle of the day, which precedes the peak expression of many JA-regulated genes at dusk.Citation7,Citation9 MYC2, which is an important transcriptional regulator of JA-mediated transcripts, is also controlled by the circadian clock and shows peak expression at dusk under non-stressed conditions.Citation10 Notably, wounding or herbivory often induces JA levels by several orders of magnitude when compared with their basal levels.Citation11 Therefore it is unlikely that the non-induced, basal fluctuations of JA represent a relevant measure of the plant’s diurnal defensive state. Inducing plants at different times during the day and night and measuring their defensive states might provide a more realistic estimation of the diurnal variation in defenses. Arimura and colleagues showed that nocturnal damage to lima bean leaves increased JA levels 2–3 times higher when compared with treatments during the day; notably, when leaves were induced by continuous herbivore feeding JA levels remain continuously high and do not show a diurnal rhythm.Citation12 In contrast, Trichoplusia ni-attacked A. thaliana plants still accumulate JA in a diurnal pattern,Citation9 suggesting that the diurnal regulation of JA might be plant and/or herbivore-specific. Arabidopsis plants treated with methyl-JA revealed that a plant’s sensitivity to JA is highest at dawn, which correlates with the peak expression levels of the JA-receptor COI1.Citation10 COI1-expression shows a similar pattern in herbivory-elicited Nicotiana attenuata leaves, with highest expression at dawn, although diurnal differences in JA sensitivity were not found in this plant.Citation13 In N. attenuata, several herbivory-induced defense metabolites and their precursors follow circadian regulation, including diterpene glycosides and phenylpropanoid-polyamine conjugates.Citation13 However, the peak accumulation of the compounds after simulated herbivory varies between local and systemic tissues, leaves and roots.
Extrafloral nectars (EFNs) are produced by some plants as indirect defense against herbivores. Interestingly, the peak diurnal production of EFNs varies between different species.Citation14,Citation15 In lima bean, extrafloral nectars are usually produced during the night, however, JA-treatments shift the pattern of EFN secretions, leading to higher production during the day.Citation16 Whether this depends on the plants circadian clock or whether it is correlated with the activity of the parasitoids remains unknown. Another indirect defense response against herbivores is the emission of volatiles. Diurnal variation in herbivory-induced volatile production was shown in corn, cotton and lima bean and probably depends on light regimes and not the circadian clock.Citation12,Citation17,Citation18
Plant defense responses to pathogen elicitation were also shown to follow diurnal patterns that are under the control of the plant’s circadian clock. Pathogen-associated molecular pattern (PAMP)-induced callose deposition in Arabidopsis was significantly higher when plants are induced in the morning, when compared with inductions in the evening, a response that depends on the functional clock component CCA1 (CIRCADIAN CLOCK ASSOCIATED 1).Citation19 This response is probably coordinated by the higher expression of PAMP perception and signaling components in the morning.Citation19 Wang and colleagues found that a number of genes involved in pathogen-induced defense responses contain clock-related elements in their promotors and show rhythmic expression patterns.Citation20 Taken together, these examples demonstrate that the levels of various defense-related processes are not homogenously expressed, but rather fluctuate in diurnal rhythms and are partly controlled by the circadian clock.
Diurnal Fluctuations in Biotic Stress
The ODH states that tissue defense should be correlated with the probability of biotic stress. Only few examples have shown that this is the case. Goodspeed and colleagues demonstrated that T. ni feeding activity on artificial diet is under circadian control and that this activity correlates with diurnal accumulations of JA in A. thaliana leaves: both peak during the day. When plants’ and insects’ rhythms were out of phase, T. ni gained more mass and caused more damage on A. thaliana, compared with plants synchronized with the activities of the insects.Citation9 However, the activity of T. ni feeding on the plant was unfortunately not described, which could be different when compared with the feeding rhythms of T. ni reared on non-responsive artificial diets. As pointed out by Jander (2012), analyzing the susceptibility of other day active herbivores to the circadian control of JA-related defenses might allow one to draw general conclusions about the evolution of a daytime defense priority against herbivores in the model plant A. thaliana.Citation21 In addition, analyzing the role of circadian clock in gating defenses against natural attackers outside the lab, in the plant’s environment, would reveal the relevance of experiments performed under these controlled conditions. We found that Arabidopsis (Col-0) plants grown in the natural environment in Germany (Thuringia) and USA (Michigan) were mainly attacked by slugs that showed nocturnal activity patterns (Stefan Meldau, Julia Kästner, Natacha Bodenhausen, unpublished data). Since slugs and plants are both rhythmic-behaving organisms, this feeding behavior is likely an interplay between the clock-driven behaviors of both organisms. Considering the current model of a priority to defend against day-active herbivores, the resistance of plants like A. thaliana might be less optimal against herbivores, such as slugs, which have largely nocturnal activity patterns. Whether A. thaliana or other plants can adjust their circadian-gated defenses to the activity of a particular attacker or whether plants prioritize their responses when attacked by both day active and nocturnal herbivores remain essential unresolved questions.
Leaf pathogens often attack at dawn when temperature and moisture conditions support their infection. Hyaloperonospora arabidopsidis, an obligate biotrophic oomycete pathogen, which causes downy mildew disease on Arabidopsis leaves, sporulates at night and disseminates its spores at dawn.Citation22 The plant’s resistance against H. arabidopsidis correlates with the probability of attack: susceptibility was lowest during dawn and increased at dusk, a phenotype that was dependent on a functional plant circadian clock.Citation20 In contrast, Arabidopsis plants inoculated with Pseudomonas syringae DC3000 were more susceptible at dawn when compared with an evening inoculation time, a phenotype that depends on the circadian clock component TIC, which is involved in regulating JA signaling.Citation10 Bhardwaj et al. (2011), found that P. syringae growth was lowest when plants where infected in the day and highest in the night. Whether the infection success of P. syringae in the natural environment correlates with results obtained from controlled inoculation experiments should be verified.Citation19 Clearly, more studies are required to identify circadian-controlled pathogen infection rhythms, preferably by analyzing their behavior under natural conditions.
Diurnal Changes of Tissue Values
The third assumption of the ODH, namely that defense states of tissues should be correlated with their “value,” mostly measured in terms of plant fitness (e.g., seed production), has to our knowledge not been tested with regard to the short-term circadian patterns. It is clear that the production and allocation of plant metabolites needed for growth and reproduction is strictly regulated by the circadian clock. The working period of the photosynthetic machinery, the opening hours of stomata or the time of starch turnover are only a few examples of clock-controlled processes important for plant resource acquisition.Citation23,Citation24
Major phytohormone pathways important for plant growth and development are also regulated by the clock. For example, plant growth promotion during the night phase is known to be regulated by rhythmic auxin and gibberrellin signaling.Citation6,Citation25-Citation27 Shin et al. (2012) discuss the circadian peak of JA responsiveness in the morning as a way of saving costly resources needed to promote plant growth in the evening. This assumption is in line with the peak of pathogen-related immune signaling at dawn.Citation19,Citation20 Whether the fitness consequences of attack for the plant change with different diurnal attack times is a central question for this analysis. For example, loss of tissues during the main growth period in the night might impose a higher fitness cost when compared with loss of the same tissue during the day. Therefore, restricting pathogen or herbivore activities to the daytime might help the plant minimizing such fitness costs.Citation10
We examined if simulated herbivory (leaf removal) in the morning or evening differentially influenced plant growth and fitness in N. attenuata. We removed leaves growing at the same nodal positions from different plants either at 9 a.m. or 7 p.m. over a period of three weeks when plants had started to elongate (35 d after germination), until all leaves were removed (). Leaf removal was alternated (e.g., 9 a.m., 7 p.m., 7 p.m., 9 a.m.) to avoid that one batch of plants benefited from having leaves longer than the other batch. Our results show that leaf removal strongly reduced plant growth and fitness, measured as plant height and seed capsule production, respectively, 65 d after germination, when compared with plants from which no leaves were removed (). Interestingly, plant height and seed capsule production was significantly lower after leaves were excised in the morning, compared with plants suffering leaf loss in the evening (). Evening-treated plants produced twice as many seed capsules and plant height was more than 10% higher when compared with morning clipped plants (). These data demonstrate that the time of attack affects a plant’s fitness and growth response.
Figure 1. Daytime of leaf removal determines plant growth and fitness costs. (A) Rosette-staged Nicotiana attenuata plants kept untreated (control) or leaves were successively removed in the morning or evening over a period of three weeks. One rosette leaf was removed each day, starting with the oldest leaves, until all rosette leaves were removed. Stem leaves were removed over three consecutive days, starting with oldest rosette leaves. Leaf removal was finished 55 d after germination. Plant height and seed capsule production was measured 65 d after germination (indicated with asterics). Plant growth conditions are described in reference Citation29. (B) Picture of representative plants 65 d after germination. (C) Plant height and number of seed capsules 65 d after germination. Small letters represent statistically significant differences between treatment groups. (One-way ANOVA, p < 0.05; Turkey HSD, n = 10).
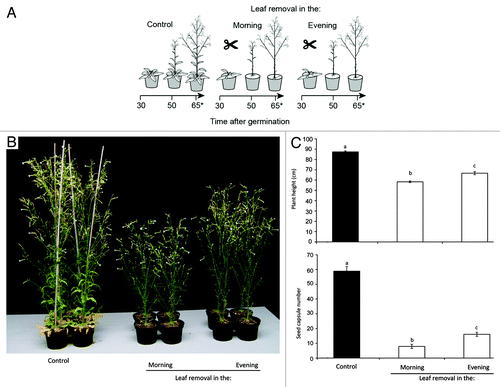
These data contrast with the predictions of Shin et al. (2012), who proposed that leaves might be best defended in the morning since this might save resources required for growth during the night. As discussed before, COI1 expression is highest at dawn in N. attenuata,Citation13 suggesting that the defenses activated through the JA pathway might be more pronounced in the morning when compared with evening treatments. Accordingly, in morning damaged plants, the induced JA-pathway might have caused higher COI1-dependent investments into defense metabolites in the remaining tissues, compared with the defense investments of plants cut in the evening. Since the JA pathway is costly for plant growth and fitness in N. attenuata,Citation28 the potential differences in the intensities of JA-mediated defenses might explain our growth and fitness data. Analyzing JA-mediated defenses after induction at different times during day and night as well as using plants with impaired JA biosynthesis or perception is required to test this hypothesis. Additionally, non-invasive experimental setups might provide a more nuanced analysis of the values of plant tissues for growth and fitness.
Conclusions
Data collected during the last four decades have revealed that plants coordinate their defenses to maximize resistance against biotic stress and limit fitness trade-offs imposed by activation of costly defense pathways. Here we discuss if the circadian regulation of defenses has been evolved to coordinate “optimal” diurnal defense against herbivores and pathogens and to reduce the associated costs for plant growth and fitness. It is clear that the circadian clock is important for synchronizing defense responses to the time of attack, however, the importance of this regulation for plant growth and fitness is unknown. The data presented here suggested that the time of attack can significantly affect plant growth and fitness and further research is needed to identify the underlying mechanisms. Most data on circadian-regulated defense pathways stem from studies performed under controlled conditions in the glasshouse or in growth chambers and are unlikely to reflect the role of the circadian clock in orchestrating plant growth, defense and susceptibility to attackers under seasonal perturbations in a plants natural environment. Performing experiments with plants with altered circadian clock components in their natural environments will reveal the real role of the circadian clock in regulating optimal defense patterns.
| Abbreviations: | ||
| ODH | = | optimal defense hypothesis |
| JA | = | jasmonic acid |
Acknowledgments
We thank Martin Schäfer for experimental support and Christoph Brütting for providing parts of the graphics used in Figure 1A. The work of Stefan Meldau is funded by Advanced Grant No 293926 of the European Research Council to Ian Baldwin. Ian Baldwin is funded by the Max-Planck-Society.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Meldau S, Erb M, Baldwin IT. Defence on demand: mechanisms behind optimal defence patterns. Ann Bot 2012; 110:1503 - 14; http://dx.doi.org/10.1093/aob/mcs212; PMID: 23022676
- Stamp N. Out of the quagmire of plant defense hypotheses. Q Rev Biol 2003; 78:23 - 55; http://dx.doi.org/10.1086/367580; PMID: 12661508
- McKey D. Adaptive patterns in alkaloid physiology. Am Nat 1974; 108:305 - 20; http://dx.doi.org/10.1086/282909
- Pokhilko A, Fernández AP, Edwards KD, Southern MM, Halliday KJ, Millar AJ. The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Mol Syst Biol 2012; 8:574; http://dx.doi.org/10.1038/msb.2012.6; PMID: 22395476
- Wenden B, Kozma-Bognár L, Edwards KD, Hall AJW, Locke JCW, Millar AJ. Light inputs shape the Arabidopsis circadian system. Plant J 2011; 66:480 - 91; http://dx.doi.org/10.1111/j.1365-313X.2011.04505.x; PMID: 21255161
- McClung CR, Davis SJ. Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Curr Biol 2010; 20:R1086 - 92; http://dx.doi.org/10.1016/j.cub.2010.10.035; PMID: 21172632
- Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol 2008; 9:R130; http://dx.doi.org/10.1186/gb-2008-9-8-r130; PMID: 18710561
- Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 2012; 17:250 - 9; http://dx.doi.org/10.1016/j.tplants.2012.01.003; PMID: 22305233
- Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc Natl Acad Sci USA 2012; 109:4674 - 7; http://dx.doi.org/10.1073/pnas.1116368109; PMID: 22331878
- Shin J, Heidrich K, Sanchez-Villarreal A, Parker JE, Davis SJ. TIME FOR COFFEE represses accumulation of the MYC2 transcription factor to provide time-of-day regulation of jasmonate signaling in Arabidopsis. Plant Cell 2012; 24:2470 - 82; http://dx.doi.org/10.1105/tpc.111.095430; PMID: 22693280
- Schäfer M, Fischer C, Meldau S, Seebald E, Oelmüller R, Baldwin IT. Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiol 2011; 156:1520 - 34; http://dx.doi.org/10.1104/pp.111.173567; PMID: 21546453
- Arimura GI, Köpke S, Kunert M, Volpe V, David A, Brand P, et al. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol 2008; 146:965 - 73; http://dx.doi.org/10.1104/pp.107.111088; PMID: 18165324
- Kim SG, Yon F, Gaquerel E, Gulati J, Baldwin IT. Tissue specific diurnal rhythms of metabolites and their regulation during herbivore attack in a native tobacco, Nicotiana attenuata. PLoS ONE 2011; 6:e26214; http://dx.doi.org/10.1371/journal.pone.0026214; PMID: 22028833
- Radhika V, Kost C, Bartram S, Heil M, Boland W. Testing the optimal defence hypothesis for two indirect defences: extrafloral nectar and volatile organic compounds. Planta 2008; 228:449 - 57; http://dx.doi.org/10.1007/s00425-008-0749-6; PMID: 18493790
- Raine NE, Willmer P, Stone GN. Spatial structuring and floral avoidance behavior prevent ant-pollinator conflict in a Mexican ant-acacia. Ecology 2002; 83:3086 - 96
- Radhika V, Kost C, Mithöfer A, Boland W. Regulation of extrafloral nectar secretion by jasmonates in lima bean is light dependent. Proc Natl Acad Sci USA 2010; 107:17228 - 33; http://dx.doi.org/10.1073/pnas.1009007107; PMID: 20855624
- Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci USA 1994; 91:11836 - 40; http://dx.doi.org/10.1073/pnas.91.25.11836; PMID: 11607499
- Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 1995; 92:4169 - 74; http://dx.doi.org/10.1073/pnas.92.10.4169; PMID: 7753779
- Bhardwaj V, Meier S, Petersen LN, Ingle RA, Roden LC. Defence responses of Arabidopsis thaliana to infection by Pseudomonas syringae are regulated by the circadian clock. PLoS ONE 2011; 6:e26968; http://dx.doi.org/10.1371/journal.pone.0026968; PMID: 22066021
- Wang W, Barnaby JY, Tada Y, Li H, Tör M, Caldelari D, et al. Timing of plant immune responses by a central circadian regulator. Nature 2011; 470:110 - 4; http://dx.doi.org/10.1038/nature09766; PMID: 21293378
- Jander G. Timely plant defenses protect against caterpillar herbivory. Proc Natl Acad Sci USA 2012; 109:4343 - 4; http://dx.doi.org/10.1073/pnas.1201443109; PMID: 22378647
- Donofrio NM, Delaney TP. Abnormal callose response phenotype and hypersusceptibility to Peronospoara parasitica in defence-compromised arabidopsis nim1-1 and salicylate hydroxylase-expressing plants. Mol Plant Microbe Interact 2001; 14:439 - 50; http://dx.doi.org/10.1094/MPMI.2001.14.4.439; PMID: 11310731
- Chen C, Xiao YG, Li X, Ni M. Light-regulated stomatal aperture in Arabidopsis. Mol Plant 2012; 5:566 - 72; http://dx.doi.org/10.1093/mp/sss039; PMID: 22516479
- Graf A, Smith AM. Starch and the clock: the dark side of plant productivity. Trends Plant Sci 2011; 16:169 - 75; http://dx.doi.org/10.1016/j.tplants.2010.12.003; PMID: 21216654
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, et al. Rhythmic growth explained by coincidence between internal and external cues. Nature 2007; 448:358 - 61; http://dx.doi.org/10.1038/nature05946; PMID: 17589502
- Rawat R, Schwartz J, Jones MA, Sairanen I, Cheng YF, Andersson CR, et al. REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proc Natl Acad Sci USA 2009; 106:16883 - 8; http://dx.doi.org/10.1073/pnas.0813035106; PMID: 19805390
- Arana MV, Marín-de la Rosa N, Maloof JN, Blázquez MA, Alabadí D. Circadian oscillation of gibberellin signaling in Arabidopsis. Proc Natl Acad Sci USA 2011; 108:9292 - 7; http://dx.doi.org/10.1073/pnas.1101050108; PMID: 21576475
- Meldau S, Ullman-Zeunert L, Govind G, Bartram S, Baldwin IT. MAPK-dependent JA and SA signalling in Nicotiana attenuata affects plant growth and fitness during competition with conspecifics. BMC Plant Biol 2012; 12; PMID: 22269060
- Krugel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 2002; 12:177 - 83; http://dx.doi.org/10.1007/PL00012666