Abstract
We recently reported that the Medicago WOX gene, STENOFOLIA (STF), acts as a transcriptional repressor in regulating leaf blade outgrowth. By using the Nicotiana sylvestris bladeless lam1 mutant as a genetic tool, we showed that the WUS-box, which is conserved among WUS clade WOX genes, is partly responsible for the repressive activity of STF. All members of the modern/WUS clade genes (WUS, WOX1-WOX7) in Arabidopsis that contain intact WUS-box can substitute for STF/LAM1 function while the intermediate and ancient clade members including WOX9,WOX11 and WOX13 cannot, due to lack of the intact WUS-box. Taken together, our results reveal a conserved repression mechanism playing a central role in cell proliferation conferred to the evolutionarily dynamic WOX gene family with acquisition of a repressor domain.
This commentary is based on our recent report in Proceedings of the National Academy of Sciences of USACitation20 and may, for purposes of discussion, include some phrases used in the original article.
Leaves are the primary organs of photosynthesis in plants where solar energy and carbon dioxide from the atmosphere are assimilated into chemical energy which supports life on earth. The mechanism of leaf polarity specification and flat lamina formation has long been of interest to biologists. Although polarity patterning along the upper/lower surface of the leaf blade through mutually antagonistic interactions of adaxial and abaxial polarity factors is required for blade flattening,Citation1-Citation7 the extent to which polarity regulates blade outgrowth is unclear, and this has been variable among plant species.
One of the classical and most severe leaf blade mutants, bladeless lam1, in Nicotiana sylvestris, is found to be caused by a mutation in a WUSCHEL-related homeobox (WOX) family transcription factor gene, LAM1/STF.Citation8 WOX family transcription factors are plant-specific and are known for their crucial roles in several developmental programs from apical-basal patterning of the embryo to stem cell maintenance and development of lateral organs. In maize, double mutation of two closely related WOX genes, narrowsheath1 and 2 (ns1 and ns2), leads to a leaf margin deletion phenotype.Citation9,Citation10 Mutation in the Arabidopsis homolog of NS1 and NS2, prs, affects lateral sepals and stipules.Citation11,Citation12 In Arabidopsis, the prs single mutant needs to be combined with a related wox1 mutant to cause a distinct narrow leaf phenotype in the wox1 prs double mutant.Citation13,Citation14 In other dicots, including petunia, Medicago, tobacco, pea and Lotus, mutation in WOX1 homologs causes a narrow blade phenotype and a floral organ defect as a single mutant.Citation8,Citation14,Citation15 The reason for this deviation is not known, but wox1 appears to be the exception in dicots. Recent study in Arabidopsis showed that WOX1 and PRS can coordinate adaxial-abaxial patterning.Citation13,Citation16 However, the polarity defects in the stf and lam1 mutants are modest, but the leaf blade and floral organ defects are severe and often stronger than single polarity mutants. Thus, whether coordination of polarity is the only mechanism by which STF/LAM1 orchestrates its blade outgrowth functions remains to be shown.
STF mainly acts as a repressor in promoting leaf blade outgrowth
STF and LAM1 are interchangeable in M. truncatula and N. sylvestris.Citation8,Citation17,Citation18 In the classical lam1 mutant, the leaf blade phenotype is very dramatic in which blades are reduced to vestigial strips that lack mesophyll differentiation.Citation18,Citation19 We made use of this severe lam1 blade phenotype and the simplicity of tobacco transformation as an ideal system for investigating the molecular mechanism of leaf blade morphogenesis.
Transient luciferase expression assay in Arabidopsis protoplast indicated that the STF protein exhibits strong repressive activity.Citation20 Mutations in the WUS-box of STF (STFm1) partially relieved this repression suggesting that STF is a transcriptional repressor through its WUS-box. Complementation analysis of the lam1 mutant showed that mutation in the WUX-box decreases the degree of complementation. Consistent with this, while fusion of repressor domain SRDX to STFm1 rescued the lam1 mutant, fusion of activator domain VP16 worsened the lam1 phenotype,Citation20 confirming that STF acts as a repressor to promote leaf blade outgrowth.
Functional conservation among WOX family proteins
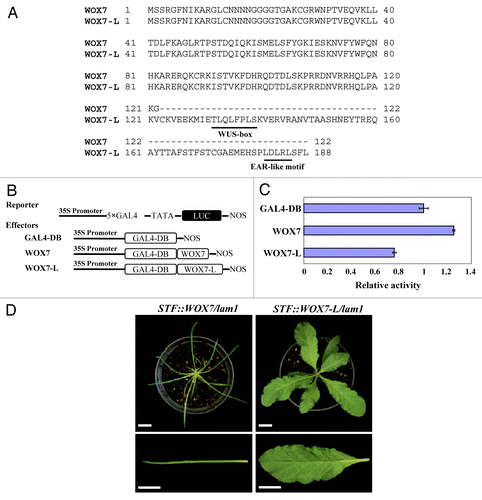
WOX genes perform crucial functions in several key plant developmental programs.Citation9,Citation14,Citation21-Citation23 While WUS and WOX5 are required for stem cell maintenance in the shoot and root apical meristems, respectively, WOX3 is required for development of lateral sepals and stipules. WUS was shown to complement both wox5 and wox3 mutants,Citation11,Citation24 suggesting a conservation of function among WOX genes. In M. truncatula and N. sylvestris, the Arabidopsis WUS can also complement the stf and lam1 leaf blade mutant phenotypes.Citation8,Citation20 We found a general mechanism for this functional conservation. In the three clade phylogenetic classification of WOX family (ancient, intermediate and modern/WUS),Citation25 functional conservation exists between members of the modern/WUS clade. This is because the WUS clade members may mainly act as transcriptional repressors for their respective functions. At least in leaf blade outgrowth, WUS and WOX1-WOX6 can act as repressors and can substitute for STF function in complementing the lam1 mutant provided that they are expressed under the control of the STF promoter.Citation20 All of the WUS clade members contain a conserved intact WUS-box which confers repressive potential. The intermediate and ancient clade WOX members, on the other hand, have partial or no WUS-box and probably act as activators. As a result, these genes cannot substitute for functions of WUS clade members. Thus, we have uncovered the extent of functional conservation and the minimum requirement for such conservation in WOX genes. The only WUS clade gene that doesn’t fit to this conclusion was WOX7 as it neither has a WUS-box nor it complements lam1, but this was found to be due to the fact that the annotation of WOX7 has only the shorter version. We identified by 3′ RACE that a longer splice variant exists, and this longer sequence (WOX7-L) has a WUS-box (). WOX7-L has repressive activity in luciferase assays and can complement lam1 albeit wavy margins ().
Figure 1. Two splice variants of Arabidopsis WOX7 and repressive activity of the WOX7-L protein. (A) Amino acid sequence alignment for WOX7 and WOX7-L. The conserved WUS-box and EAR-like motif are underlined. (B) Constructs used in transient expression assays. (C) Relative luciferase activities using WOX7 or WOX7-L as effectors compared with GAL4-DB control. Error bars indicate SD (n = 3). (D) Transgenic lam1 plants complemented with STF::WOX7 (left), STF::WOX7-L (right). Scale bars: 2 cm.
Since promotion of cell proliferation without differentiation is essential for stem cell maintenance, and repressive activity is shown to be required for WUS function in shoot apical meristem,Citation26 it is likely that STF and WUS use a similar basic mechanism of action for their respective functions in the leaf margin and shoot apical meristem, respectively. The fact that both WUS and STF act as transcriptional repressors for their core functions, and WUS can substitute for STF at the adaxial-abaxial junction in the middle mesophyll and leaf margin, suggests that there are dispersed mersitematic cells in the determinate leaf primordia that generate new cells during leaf morphogenesis analogous to the SAM. Alternatively, the proliferating cells in leaf primordia may not have or have lost meristematic ontogeny as such, but WUS and STF may have the capacity to convert ordinary cells into meristematic cells wherever they are expressed. This is consistent with the long established observation that plant cells are plastic in which cells from even adult tissues can be made to dedifferentiate into callus by cultivating them on culture media with appropriate concentration of phytohormones. This is particularly appealing because both WUS and STF are supposed to modulate hormonal signals.Citation8,Citation27,Citation28 Nevertheless, the end result of WUS activity in the SAM is continuous generation of undifferentiated cells, whereas that of STF is symmetrically flattened blade outgrowth. We propose that expression pattern and tissue context may account for these different outcomes. WUS is discretely expressed in few cells at the organizing center of the SAMCitation23,Citation28-Citation30 so long as the SAM exists, whereas STF is dynamically expressed in patches at the adaxial-abaxial junction in a developmentally regulated manner, disappearing altogether as the leaf matures.Citation8 WUS expression in the SAM is several cells away from differentiated cells or factors acting in cell differentiation insulated by KNOX genes, and the newly formed cells may retain their undifferentiated nature until they reach to the primordial initiation site, whereas STF expression is bordered by adaxial and abaxial factors, perhaps just one cell layer away on either side. Thus, new cells formed by the action of STF are likely to quickly acquire adaxial or abaxial identity, accounting for differentiated blade growth. Consistent with this hypothesis, expression of WUS under the control of the STF promoter in the lam1 mutant drives blade outgrowth similar to STF,Citation8,Citation20 rather than maintenance of undifferentiated stem cells.
However, this does not necessarily mean that the only difference between WUS, STF and other WUS clade WOX genes is promoter and tissue context. It should be noted that complementation of the lam1 mutant with WUS or other WUS clade genes is not perfect. Although all WUS clade genes can rescue lam1 when expressed under the STF promoter, at least at later stages of development, most of the blades including those complemented by WUS show wavy margins. There are differences in the structures of the WUS and STF proteins. The STF protein contains a 60 amino acids (aa) extension at the N-terminus which is missing in WUS, and a 10 aa highly conserved STF-box at the C-terminus is replaced with an EAR-like motif in the WUS protein. In fact, Sequence alignment indicates that WUS and STF share approximately 70% aa identity only at the homeodomain region where the highest homology exists. So, the most likely mechanism for conservation of function is the capacity to share some transcriptional targets where both the WUS and STF homeodomains can bind. In this scenario, there would be some targets that are regulated by both WUS and STF which are required for the basic cell proliferation activity, but there would also be other specific targets and interaction partners required for fine tuning of activity which may not be shared by WUS and STF. Future experiments will identify which transcriptional targets will be shared by STF and WUS or other WUS clade members.
Maize NS1 complements the lam1 mutant with additional phenotypes
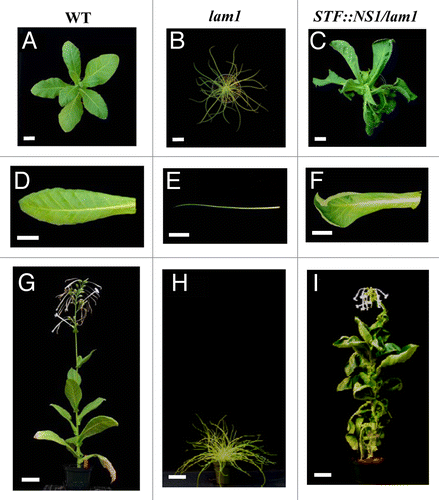
Because all of the Arabidopsis WUS clade genes including WOX3 can complement the lam1 mutant, and monocots usually have narrower leaves, we wondered how far this complementation can stretch between dicots and monocots. There is no obvious STF homolog in monocots,Citation8,Citation17 but maize NS1 and NS2 are homologs of WOX3 belonging to the WUS clade that mediate blade outgrowth. To test if monocot WUS clade genes can substitute for STF function, we introduced maize NS1 into the lam1 mutant under the control of the STF promoter. We found that all of the lam1 leaves are complemented, but most of the lines showed upward curling leaves and twisted stems (). These observations suggest that although the NS1 gene is recognized in the STF-mediated dicot leaf developmental pathway, some fine tuning is missing more so in NS1 than other WUS clade dicot genes. The reason for these additional phenotypes is not known. One possibility could be that NS1 is less sensitive to factors that regulate STF activity, and becomes more active in the lam1 plants. The curling phenotype is a reminiscent of STF overexpression in wild-type N. sylvestris.Citation8 If STF activity is posttranscriptionally and posttranslationally regulated, the insensitivity of NS1 to such regulatory mechanisms, due to the monocot-dicot divergence, may result in more active NS1. Further experiments are needed to evaluate such speculations. Taken together, these complementation experiments suggest that the basic mechanism of leaf blade outgrowth is shared by WUS clade WOX genes and this is conserved in both dicots and monocots. However, this conservation refers to the basic mechanism and does not imply that WUS clade WOX genes are redundant in function. This is obvious at least for leaf blade development since strong stf and lam1 single mutant phenotypes wouldn’t be observed if any of the WOX genes were redundant. Arabidopsis appears to be the exception in this regard where WOX1 and PRS redundantly control blade outgrowthCitation13,Citation14 although PRS has additional specific functions in the flower.Citation11,Citation12 Similarly, since we haven’t transformed lam1 with genomic NS1 and ns1 ns2 with genomic STF or LAM1, we cannot conclude at this stage that the monocot and dicot genes complement each other. What we are suggesting is that transcriptional repression is perhaps the basic mechanism for the function of WUS clade WOX genes and this is conserved even between dicots and monocots. How WOX-mediated repression promotes cell proliferation in stem cell maintenance and blade outgrowth is an important question for future consideration which will enlighten our understanding of a fundamental mechanism in plant development.
Figure 2. Functional complementation of lam1 mutant by maize NS1 CDS. (A, D and G) Wild type tobacco plant and leaf. (B, E and H) lam1 mutant plant and leaf. (C, F and I) Transgenic plant and leaf of lam1 mutant transformed with STF::NS1. Top panel, young plants; bottom panel, mature plants. Scale bars: 5 cm.
Acknowledgments
We thank Dr Michael Scanlon for providing Maize B73 seeds. This work was supported by the Oklahoma Center for the Advancement of Science and Technology under grant PBS11–002 and in part by the National Science Foundation under Grant EPS-0814361.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Husbands AY, Chitwood DH, Plavskin Y, Timmermans MC. Signals and prepatterns: new insights into organ polarity in plants. Genes Dev 2009; 23:1986 - 97; http://dx.doi.org/10.1101/gad.1819909; PMID: 19723761
- Tsukaya H. Mechanism of leaf-shape determination. Annu Rev Plant Biol 2006; 57:477 - 96; http://dx.doi.org/10.1146/annurev.arplant.57.032905.105320; PMID: 16669771
- Iwakawa H, Ueno Y, Semiarti E, Onouchi H, Kojima S, Tsukaya H, et al. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol 2002; 43:467 - 78; http://dx.doi.org/10.1093/pcp/pcf077; PMID: 12040093
- Bowman JL, Eshed Y, Baum SF. Establishment of polarity in angiosperm lateral organs. Trends Genet 2002; 18:134 - 41; http://dx.doi.org/10.1016/S0168-9525(01)02601-4; PMID: 11858837
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 2001; 411:709 - 13; http://dx.doi.org/10.1038/35079635; PMID: 11395776
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature 2001; 411:706 - 9; http://dx.doi.org/10.1038/35079629; PMID: 11395775
- Waites R, Selvadurai HRN, Oliver IR, Hudson A. The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 1998; 93:779 - 89; http://dx.doi.org/10.1016/S0092-8674(00)81439-7; PMID: 9630222
- Tadege M, Lin H, Bedair M, Berbel A, Wen J, Rojas CM, et al. STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris. Plant Cell 2011; 23:2125 - 42; http://dx.doi.org/10.1105/tpc.111.085340; PMID: 21719692
- Nardmann J, Ji J, Werr W, Scanlon MJ. The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development 2004; 131:2827 - 39; http://dx.doi.org/10.1242/dev.01164; PMID: 15169755
- Scanlon MJ, Schneeberger RG, Freeling M. The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 1996; 122:1683 - 91; PMID: 8674408
- Shimizu R, Ji J, Kelsey E, Ohtsu K, Schnable PS, Scanlon MJ. Tissue specificity and evolution of meristematic WOX3 function. Plant Physiol 2008; 149:841 - 50; http://dx.doi.org/10.1104/pp.108.130765; PMID: 19073779
- Matsumoto N, Okada K. A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Genes Dev 2001; 15:3355 - 64; http://dx.doi.org/10.1101/gad.931001; PMID: 11751640
- Nakata M, Matsumoto N, Tsugeki R, Rikirsch E, Laux T, Okada K. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell 2012; 24:519 - 35; http://dx.doi.org/10.1105/tpc.111.092858; PMID: 22374393
- Vandenbussche M, Horstman A, Zethof J, Koes R, Rijpkema AS, Gerats T. Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. Plant Cell 2009; 21:2269 - 83; http://dx.doi.org/10.1105/tpc.109.065862; PMID: 19717616
- Zhuang LL, Ambrose M, Rameau C, Weng L, Yang J, Hu XH, et al. LATHYROIDES, encoding a WUSCHEL-related Homeobox1 transcription factor, controls organ lateral growth, and regulates tendril and dorsal petal identities in garden pea (Pisum sativum L.). Mol Plant 2012; 5:1333 - 45; http://dx.doi.org/10.1093/mp/sss067; PMID: 22888154
- Nakata M, Okada K. The three-domain model: A new model for the early development of leaves in Arabidopsis thaliana. Plant Signal Behav 2012; 7:1423 - 7; http://dx.doi.org/10.4161/psb.21959; PMID: 22951404
- Tadege M, Lin H, Niu L, Mysore KS. Control of dicot leaf blade expansion by a WOX gene, STF. Plant Signal Behav 2011; 6:1861 - 4; http://dx.doi.org/10.4161/psb.6.11.17761; PMID: 22057334
- McHale NA. A nuclear mutation blocking initiation of the lamina in leaves of Nicotiana sylvestris.. Planta 1992; 186:355 - 60; http://dx.doi.org/10.1007/BF00195315
- McHale NA, Marcotrigiano M. LAM1 is required for dorsoventrality and lateral growth of the leaf blade in Nicotiana. Development 1998; 125:4235 - 43; PMID: 9753678
- Lin H, Niu L, McHale NA, Ohme-Takagi M, Mysore KS, Tadege M. Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc Natl Acad Sci USA 2013; 110:366 - 71; http://dx.doi.org/10.1073/pnas.1215376110; PMID: 23248305
- Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell 2008; 14:867 - 76; http://dx.doi.org/10.1016/j.devcel.2008.03.008; PMID: 18539115
- Lenhard M, Bohnert A, Jürgens G, Laux T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 2001; 105:805 - 14; http://dx.doi.org/10.1016/S0092-8674(01)00390-7; PMID: 11440722
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998; 95:805 - 15; http://dx.doi.org/10.1016/S0092-8674(00)81703-1; PMID: 9865698
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007; 446:811 - 4; http://dx.doi.org/10.1038/nature05703; PMID: 17429400
- van der Graaff E, Laux T, Rensing SA. The WUS homeobox-containing (WOX) protein family. Genome Biol 2009; 10:248; http://dx.doi.org/10.1186/gb-2009-10-12-248; PMID: 20067590
- Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 2009; 21:3493 - 505; http://dx.doi.org/10.1105/tpc.109.069997; PMID: 19897670
- Tadege M, Mysore KS. Tnt1 retrotransposon tagging of STF in Medicago truncatula reveals tight coordination of metabolic, hormonal and developmental signals during leaf morphogenesis. Mob Genet Elements 2011; 1:301 - 3; http://dx.doi.org/10.4161/mge.18686; PMID: 22545243
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, et al. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 2005; 438:1172 - 5; http://dx.doi.org/10.1038/nature04270; PMID: 16372013
- Jönsson H, Heisler M, Reddy GV, Agrawal V, Gor V, Shapiro BE, et al. Modeling the organization of the WUSCHEL expression domain in the shoot apical meristem. Bioinformatics 2005; 21:Suppl 1 i232 - 40; http://dx.doi.org/10.1093/bioinformatics/bti1036; PMID: 15961462
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000; 100:635 - 44; http://dx.doi.org/10.1016/S0092-8674(00)80700-X; PMID: 10761929